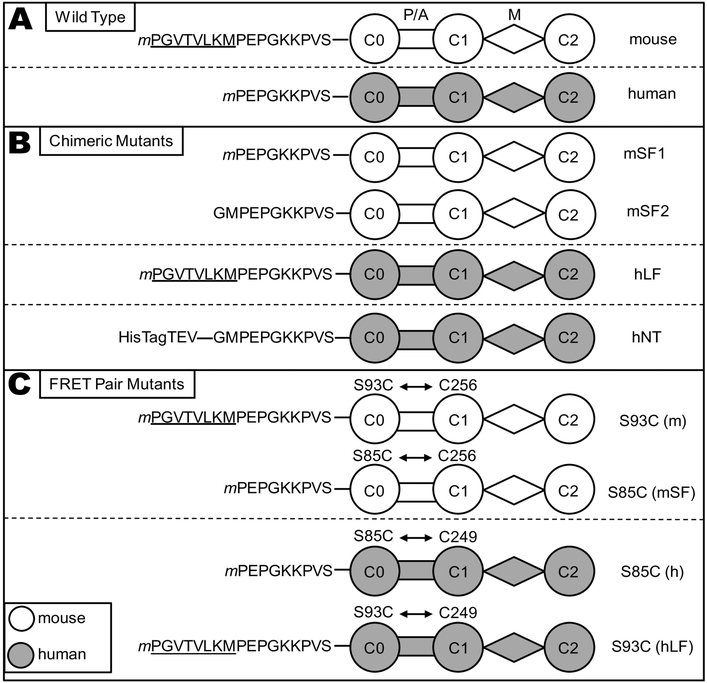
Fig. 3. C0-C2 constructs.
Schematic representation of the cMyBP-C “C0-C2” fragment constructs used in this study. (A) Wild Type C0-C2 constructs differ in their N-terminal sequence, with the mouse (white) isoform containing an N-terminal extension (NTE, underlined) relative to human (gray). All constructs, with the exception of mSF2 and hNT, (defined below) contain a TEV protease-cleavable C-terminal 6× His tag (extending from domain C2) for purification that is removed for functional and structural studies, (cleaved tags not shown). Starting methionine residues (m) were not present in the mature proteins. (B) Mouse short form (mSF1) has the 8-residue NTE removed from the N-terminus and used for actin binding and TR-FRET studies. A second mouse short form (mSF2) was constructed as an N-terminal tagged protein and the resulting protein after removal of the tag with TEV protease begins GMPEP (see methods) and used for myosin binding studies. Human long form (hLF) has the 8-residue mouse NTE added to the N-terminus. Human N-terminal tagged (hNT) C0-C2, used as an uncleaved control for myosin binding assays to non-specifically extend the N-terminus, contains a 6× His tag, 15 amino acids from the pET45 vector, and the TEV recognition sequence for a total of 28 amino acids at the amino terminus prior to GMPEP. (C) WT C0-C2 has a single endogenous Cys in domain C1 (C249 human, C256 mouse) that is readily labeled by thiol-reactive probes. A second Cys in C0 is introduced by site-directed mutagenesis (S85C in human; S93C in mouse) in order to measure FRET distances from C0 to C1. The S93C/S85C were also introduced into the mSF1/hLF chimeras for TR-FRET.