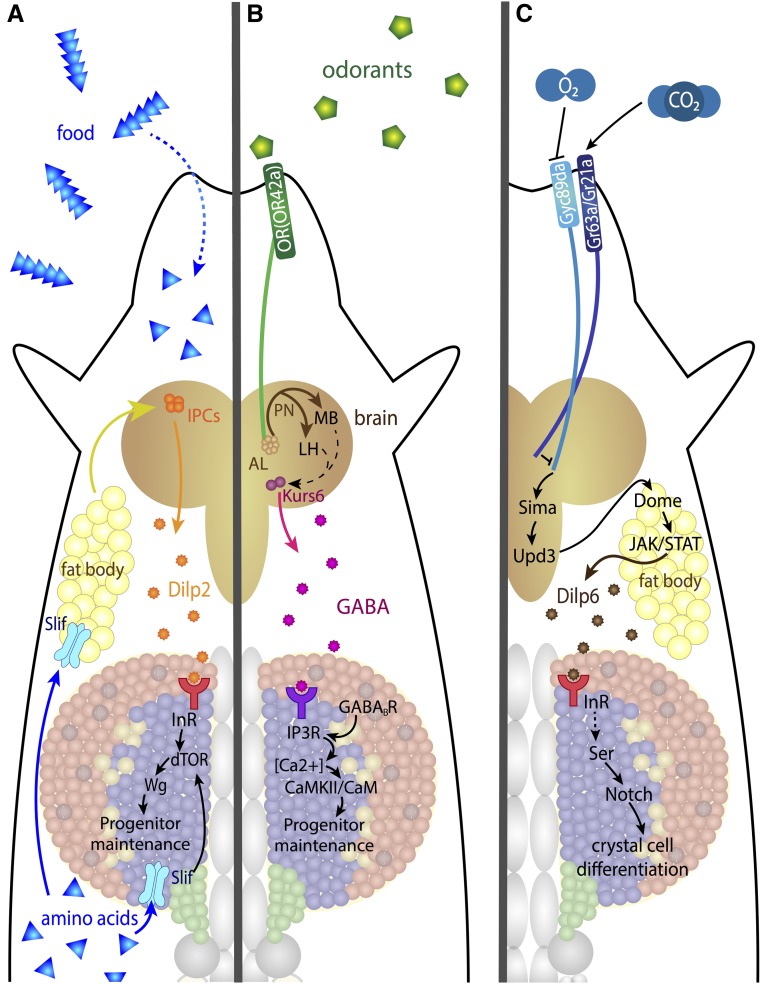
Figure 6.
Control of larval hematopoiesis by nutrition, olfaction, and hypoxia. (A) Ingested proteins are broken down into individual amino acids and absorbed into the hemolymph. The cells of the fat body and the medullary zone (MZ) express the amino acid transporter Slimfast (Slif), which allows amino acids to enter these organs. A systemic signal from the fat body then activates Dilp2-expressing insulin-producing cells (IPCs) within the brain. Secreted Dilp2 binds the Insulin receptor (InR) expressed in the MZ. Target of Rapamycin (TOR) activation downstream of InR is aided by amino acid-related signals. Wingless (Wg) functions downstream of TOR to promote progenitor maintenance. (B) Environmental odors are sensed by the OR42a+ neuron of the terminal organ, which subsequently activates a neuronal circuit that involves the antennal lobes (AL), projection neurons (PN), mushroom bodies (MB), and lateral horns (LH). This process activates Kurs6+ neurosecretory cells that secrete γ-aminobutyric acid (GABA) into the hemolymph, leading to GABABR activation in the MZ progenitors. The downstream calcium signal enables progenitor maintenance. (C) The heterodimeric receptor complex made up of Gr63a and Gr21a is activated upon CO2 binding. Normal environmental CO2 levels keep the CO2-sensing neuron active. Oxygen inhibits the atypical guanylyl cyclase, Gyc89da, and the Gyc89da+ neurons become active under hypoxic conditions. The hypoxia- and CO2-sensing neurons both project from the terminal organ to the subesophageal ganglion of the brain, where they form inhibitory synapses such that an active CO2-sensing neuron further inhibits the hypoxia-sensing neurons under normoxia. Under hypoxic conditions, activation of the hypoxia-sensing neuron leads to stabilization of Sima in a specific set of ventral nerve cord neurons, a process that ultimately results in a brain-initiated cytokine signal, Upd3, which activates Domeless (Dome) in the fat body. Downstream of this signal, Dilp6 is generated by the fat body cells, which then binds InR in lymph gland blood precursors to increase Serrate levels and therefore Notch signaling. The net result of this multiorgan signaling cascade is that, under hypoxic conditions or loss of CO2 reception, the excess Notch activation causes an increase in crystal cell number.