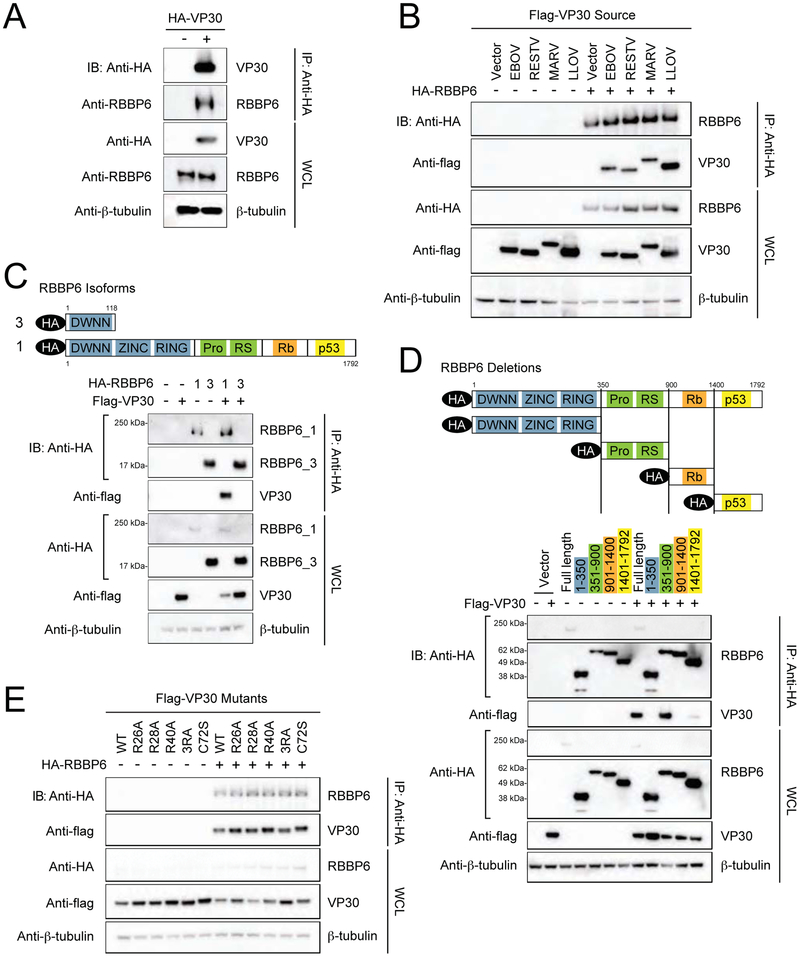
FIGURE 3. Interaction between EBOV VP30 and human RBBP6.
(A) Co-immunoprecipitation of HA-VP30 with endogenous RBBP6. HEK293T cells were transfected with HA-VP30 and immunoprecipitation (IP) was performed using anti-HA magnetic beads. Representative western blots of whole cell lysates (WCL) and eluates after IP are shown, β-tubulin was used as a loading control in WCLs. See also Supplemental Figure S1.
(B) Flag-VP30 from Zaire ebolavirus (EBOV), Reston virus (RESTV), Lloviu virus (LLOV) and Marburg virus (MARV) co-immunoprecipitate with HA-RBBP6.
(C) A schematic representation of domain organization of RBBP6 isoform 1 and 3 (top). RBBP6 functional domains consists of domain with no name (DWNN), zinc knuckle, ring finger domain, proline and arginine-serine rich domains followed by retinoblastoma (Rb) and p53 binding domains. Bottom panel depicts co-immunoprecipitation of flag-tagged VP30 with transiently expressed HA-RBBP6 isoform 1 or 3. HEK293T cells were transfected with HA-RBBP6 alone or in combination with flag-VP30, as indicated followed by IP with anti-HA.
(D) Schematic drawing of RBBP6 truncation mutants used in domain mapping studies (top). Anti-HA IP was performed after co-expression of HA-tagged full-length RBBP6 or different truncations in combination with empty vector or flag-VP30.
(E) Immunoprecipitation with anti-HA in cell lysates expressing HA-RBBP6 and flag-tagged RNA binding mutants of VP30. 3RA: R26A/R28A/R40A.