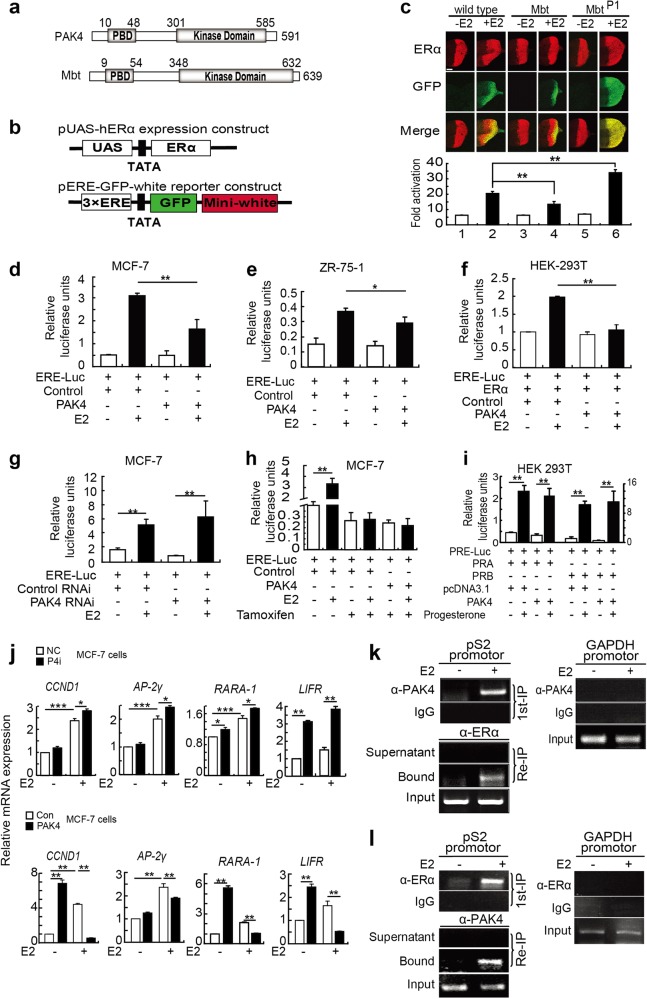
Figure 2.
PAK4 represses ERα-mediated transactivation in an E2-dependent manner. a Schematic representation of Drosophila Mbt and its ortholog in human, PAK4. The PBD and Kinase domain are positioned in the Mbt and PAK4 proteins. Numbers indicate amino-acid position. b Schematic representation of the expression and reporter constructs. The expression constructs include human ERα driven by a UAS promoter. The reporter construct harbors the GFP reporter gene controlled by the three copies of the EREs and the white reporter gene driven by the corresponding endogenous promoter. c Mbt represses ERα-induced transactivation in Drosophila. Fly lines carrying a gain-of-function mutation of Mbt (GMR-G4/ + ; UAS-Mbt/ + ) or a loss-of-function mutation of Mbt (GMR-G4/ + ; MbtP1) were crossed to two fly models expressing ERα proteins and harboring an ERE-GFP-white reporter gene in the pericentric region. The expression of ERα controlled by a GMR-GAL4 driver in the UAS-GAL4 system in eye imaginal discs of the third instar larvae was assessed by immunostaining using an anti-ERα antibody (upper panels). The effects of the Mbt and MbtP1 mutations on ERα-mediated transactivation were assessed by examining of GFP expression (middle panels). Merged images are shown in the lower panels. The quantification of GFP expression as revealed by color intensity gradations with Adobe Photoshop (histogram) is shown at the bottom. **P < 0.01. Scale bars, 50 μm. d, e The transcriptional activity of ERα was decreased by overexpression of PAK4 both in MCF-7 and ZR-75-1 cells. Cells were transiently transfected with pGL3-ERE-Luc (ERE-Luc), control and PAK4 expression plasmids as indicated in the absence or presence of ligand (E2). f The ERE luciferase reporter assay in HEK 293T cells were transfected with the expression vector encoding ERα and the indicated constructs. g Control RNAi and PAK4 RNAi were transfected into MCF-7 cells, and 24 h later, the cells were transiently transfected with pGL3-ERE-Luc. All the transfected cells were treated with or without E2 (10−9 M) for 24 h. h ERE luciferase activity in MCF-7 cells transfected with ERE-Luc and the PAK4 and control expression vectors. Cells were treated with or without 10−9 M E2 and treated with 10−6 M tamoxifen for 24 h before harvesting. i PAK4 does not affect progesterone-induced PR transcriptional activation. HEK 293T cells were co-transfected with a PRE-Luc reporter construct and an expression vector encoding PRA or PRB and PAK4. Cells were treated with or without 10−8 M progesterone for 24 h before harvesting. All the data are represented as the means ± s.e.m. **P < 0.01; *P < 0.05. j Real-time quantitative PCR (RT-qPCR) analysis showing the effect of PAK4 on activation of four ERα target genes. MCF-7 cells stably overexpressing or knocking down PAK4 were harvested after treatment with or without E2 (10−9 M) for 24 h. Total RNA was analyzed by RT-qPCR. Levels of all mRNAs were normalized to that of β-actin mRNA. Statistical significance of differences between experimental groups was assessed one-way ANOVA. Error bars represent mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001. k, l PAK4 and ERα are predominantly recruited to the ERE at heterochromatic loci in the presence of E2. MCF-7 cells were grown in medium with or without E2 (10−9 M) for 45 min. ChIP assays and ChIP/Re-IP experiments were performed using specific antibodies against PAK4 and ERα, as indicated