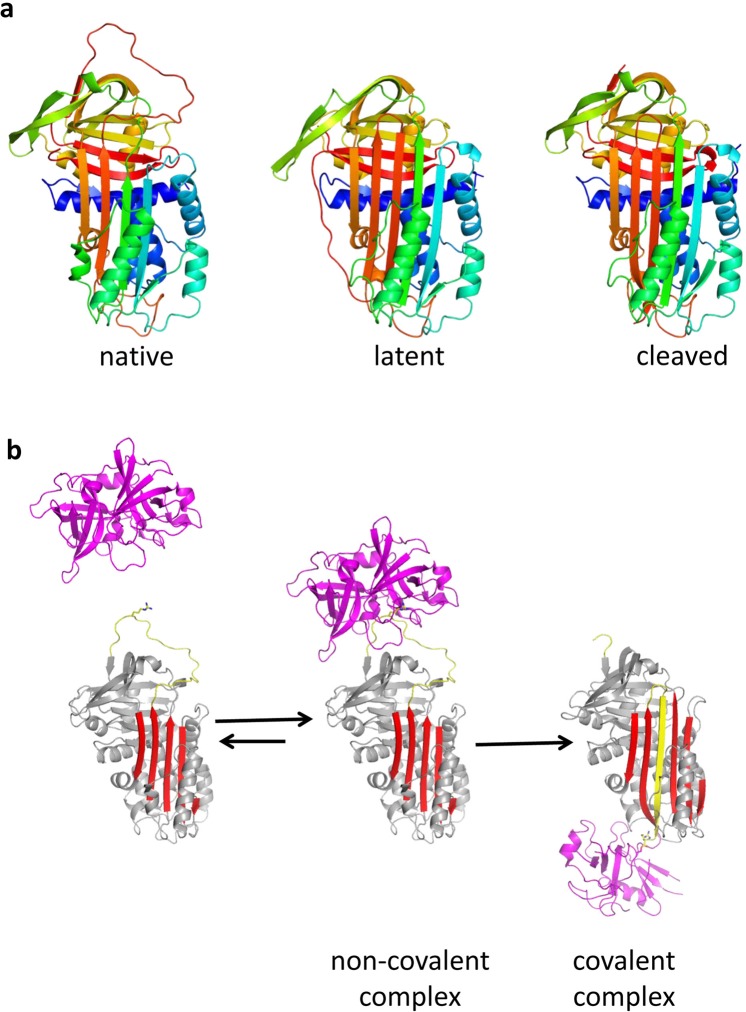
Figure 1.
Structural forms of PAI-1 and the serpin mechanism of protease inhibition: (a) PAI-1 is a conformationally labile protein and can rapidly transition from the native (left, 3pb17) to the latent (middle, 1lj5) state. Ribbon diagrams are shown coloured from N-to-C terminus (blue to red). Conversion to the latent state involves incorporation of the RCL (loop at top) into β-sheet A (front sheet) and the extension of strand 1 of β-sheet C (s1C). As with most serpins, as similar conformation is obtained upon cleavage within the RCL (right, 3cvm58). (b) Mechanism of protease inhibition by PAI-1 depicted using PDB structures 5brr8 (tPA:PAI-1) and 1ezx59 (anti-trypsin:trypsin). The elements of PAI-1 responsible for protease inhibition are the RCL (yellow, with P1 Arg depicted as sticks) and β-sheet A (red). After recognition of the RCL by a protease (magenta, centre), the protease is irreversibly translocated to the opposite pole of PAI-1 and trapped as a covalent complex (right).