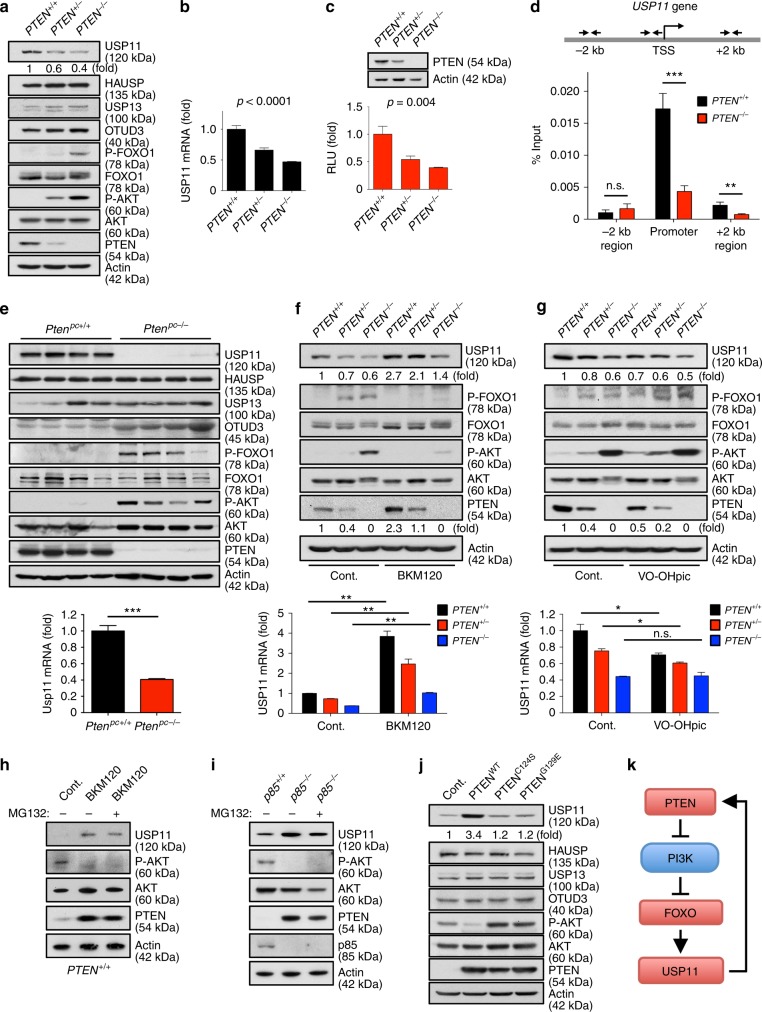
Fig. 8.
A PTEN-PI3K-FOXO-USP11 auto-regulatory feedforward mechanism. a, b Lysates and total RNAs from PTEN+/+, PTEN+/-, and PTEN-/- HCT116 cells were subjected to immunoblotting (IB) (a) and RT-qPCR (b). n = 3, p value was determined by ANOVA. (c) Luciferase reporter analysis of the USP11 promoter in PTEN+/+, PTEN+/-, and PTEN-/- HCT116 cells. n = 3, p value was determined by ANOVA. d Chromatin levels of FOXO1 at proximal promoters of human USP11 were compared between PTEN+/+ and PTEN-/- HCT116 cells by quantitative ChIP assays. Enrichment of FOXO1 was analyzed with respect to the input control (before IP) and normalized to IgG control. TSS, transcription start site. n = 3. e Lysates and total RNAs from anterior prostates of wild-type (Ptenpc+/+) and Probasin-Cre4;PtenloxP/loxP (Ptenpc-/-) mice at 11 weeks of age (n = 4) were subjected to IB (top) and RT-qPCR (bottom). f Lysates and total RNAs from PTEN+/+, PTEN+/-, and PTEN-/- HCT116 cells treated with 500 nM BKM120 for 24 h were subjected to IB (top) and RT-qPCR (bottom). n = 3. g Lysates and total RNAs from PTEN+/+, PTEN+/-, and PTEN-/- HCT116 cells treated with 500 nM VO-OHpic for 8 h were subjected to IB (top) and RT-qPCR (bottom). n = 3. h Lysates from PTEN+/+ HCT116 cells treated with 500 nM BKM120 for 24 h together with the absence or presence of MG132 (10 μM, 8 h before harvesting) were subjected to IB. i Lysates from p85+/+ and p85-/- MEFs treated with 10 μM MG132 for 8 h were subjected to IB. j Lysates from PTEN-/- HCT116 cells transfected with wild-type (WT) or phosphatase-inactive PTENC124S and PTENG129E subjected to IB. k A model for a PTEN-PI3K-FOXO-USP11 auto-regulatory feedforward mechanism. Error bars represent ± SEM. p Value was determined by Student’s t test (n.s. non-significant; *p < 0.05, **p < 0.01, ***p < 0.001). ChIP, chromatin immunoprecipitation; IP, immunoprecipitation; MEFs, mouse embryonic fibroblasts; RT-qPCR, quantitative reverse transcription PCR