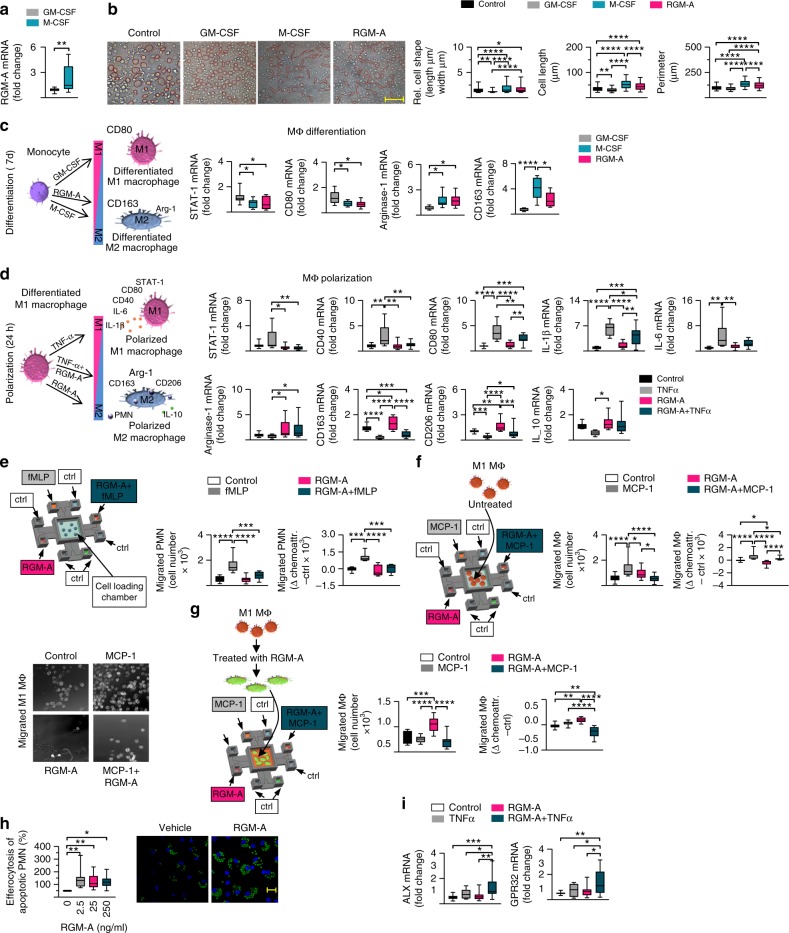
Fig. 1.
RGM-A controls macrophage phenotype and regulates human PMN/macrophage chemotaxis and chemokinesis. To mark the differentiation into the classically activated (M1) or alternatively activated (M2) phenotype, human PBMCs were stimulated with GM-CSF, M-CSF or RGM-A for 7 d, and a RGM-A transcript expression in differentiated M1 and M2 macrophages (MΦ) was quantified by RT-PCR (n = 12). b The cell morphology was analyzed by phase contrast images and measurements of the cell shape, cell length and perimeter in each high-power field (magnification ×200), Scale bar: 20 µm (n = 220). c The expression of key genes that contribute to the M1 differentiation, STAT-1 and CD80, and central genes of the M2 differentiation, Arg1 and CD163, were analyzed (n = 12). d To investigate the role of RGM-A in the phenotypic polarization of macrophages, M1 macrophages were challenged with RGM-A and subsequently with TNF-α or vehicle for 24 h. The gene expression of M1 polarization markers including STAT-1, CD40, CD80, IL-1β, and IL-6 as well as key genes of the M2 polarization such as Arg1, CD163, CD206, and IL-10 were quantified by RT-PCR (n = 14). e Schematic model of the microfluidic migration chamber. To investigate the effect of RGM-A on the regulation of e neutrophil chemotaxis (n = 10) and f, g MΦ chemotaxis/chemokinesis, chemoattractive gradients, such as N-formylmethionyl-leucyl-phenylalanine (fMLP), monocyte chemotactic protein (MCP-1) and RGM-A, were established between a range of eight peripheral wells and a central cell loading well. PMN and M1 MΦ chemotaxis were evaluated using a Casy TT cell counter (Omni Life Science, Bremen, Germany) (n = 18). f Images represent the trafficking M1 MΦ (×100 magnification). g To determine the impact of RGM-A on macrophage chemokinesis, M1 MΦ were treated with RGM-A for 10 h and the migration toward the defined gradients was evaluated using Casy TT cell counter. h The rate of MΦ clearance of human apoptotic PMNs was assessed photometrically (n = 11) and by immunofluorescence (Scale bar: 20 µm). i mRNA expression of the ALX/FPR2 and GPR32 receptors (n = 15). The results are representative of 3–8 independent experiments and are expressed as the median ± 95% CI, one-way ANOVA with Bonferroni correction (b–i), unpaired two-tailed Student’s t-test (a), *P < 0.05; **P < 0.01; ***P < 0.001, ****P < 0.0001