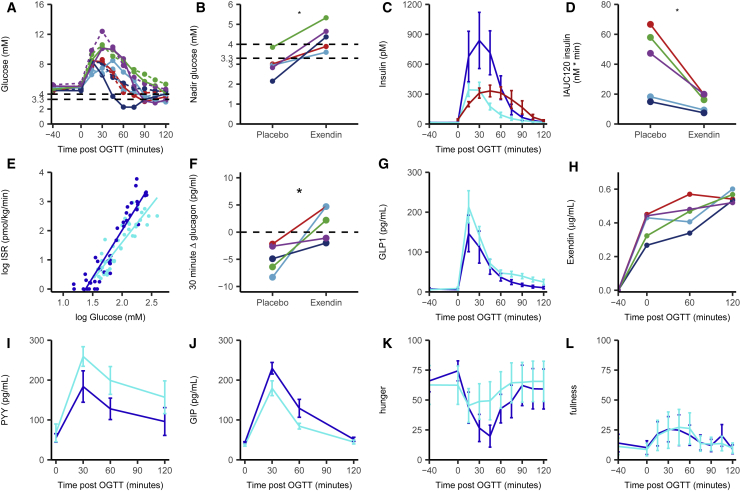
Figure 1.
Exendin-9 Infusion in Post-gastrectomy Participants Receiving a 50 g OGTT
Plasma parameters from 5 post-gastrectomy participants receiving either Exendin-9 or placebo in a cross-over design and challenged with a 50 g OGTT at time = 0.
(A) Plasma glucose levels on placebo infusion (solid lines) or Exendin-9 infusion (dotted lines). Colors indicate individual participants.
(B) Nadir glucose concentrations, taken from data shown in (A).
(C) Plasma insulin concentrations for gastrectomy patients given placebo (light blue) or Exendin-9 (dark blue) or control patients (red, control data from previous study) (Roberts et al., 2018b).
(D) Incremental area under the curve of insulin levels over 120 min. Colors represent individuals.
(E) Correlation between log insulin secretion rate (ISR) and log glucose concentration using all measured time points after the OGTT during placebo (dark blue) or Exendin-9 (light blue) infusion.
(F) Delta plasma glucagon concentrations between 0 and 30 min after the OGTT in either placebo- or Exendin-9-infused post-gastrectomy patients. Individuals are paired.
(G–L) Total GLP-1 (G), PYY (I), and GIP (J) concentrations and hunger (K) and fullness (L) ratings in placebo-infused (dark blue) or Exendin-9-infused (light blue) gastrectomy patients. Data are represented as mean ± SD. Areas under the curve between placebo and Exendin-9 are statistically different for GLP-1, PYY, and GIP, with p < 0.05 using paired Student’s t test.
(H) Exendin-9 concentrations measured during the Exendin-9 infusion.
Colors represent individuals. ∗ indicates that the two groups are statistically different with p < 0.05 using paired Student’s t test.