Abstract
Allergic asthma is becoming increasingly prevalent in the developed world, and many common allergens are capable of inducing allergic asthma responses, particularly in atopic individuals. Unmethylated CpG-oligonucleotide (ODN) therapy can shift the immune response to mitigate these allergic responses. Therapeutic and prophylactic delivery of soluble CpG-ODN in preclinical studies has shown promise in treating existing asthma and preventing allergic responses upon subsequent allergen exposure, respectively. However, when CpG-ODN is coupled with nanoparticles or self assembled into nanostructures, improved efficacy of CpG-ODN treatment for several common allergens is observed in preclinical studies and clinical trials. Here we discuss the role of CpG-ODN in treating allergic asthma and how nanoparticle-based delivery can further enhance its therapeutic properties.
Keywords: : allergic asthma, CpG-ODN, nanoparticles, prophylactic treatment, Th1 cytokines, Th2 cytokines, therapeutic treatment
Asthma is a respiratory disease that affects approximately 300 million individuals worldwide, including over 26 million individuals in the USA [1,2]. Allergic asthma is the most common form of asthma, and is a result of otherwise harmless airborne environmental allergens, or aeroallergens, triggering an excessive immune response [3,4]. Although still a matter for debate, it is generally considered that susceptibility to allergens or atopy, is due to a combination of genetic (e.g., polymorphisms in the IL-4-receptor α gene) and environmental (e.g., exposure to allergens in early childhood) factors [5], the latter potentially explaining the rising incidences of allergic asthma in the western world over the past half century [6]. The symptoms of asthma include reversible airway obstruction, airway hyperresponsiveness (AHR), excess mucus secretion and airway remodeling [7]. Treatments for asthma have traditionally consisted of inhaled anti-inflammatories and bronchodilators [8]. These are often delivered together to reduce both inflammation and to reverse airway obstruction [8]. Although such treatments are effective for many asthma sufferers, an estimated 10% of cases are not properly managed [2,7,9]. In addition, current treatments for asthma primarily target the symptoms but not the cause of the disease, thus ongoing medication is required for indefinite periods. To improve the efficacy of treating allergic asthma, new therapies must be developed that target the cause of the disease which, in atopic individuals, is the generation of unnecessary polarized immune responses to particular allergens, with the ultimate aim being to deliver a safe therapy that provides effective and sustained protection from allergic asthma that also encourages patient compliance.
It is well recognized that allergic asthma results from the production of IgE antibodies with specificity for an inhaled allergen, along with the generation and recruitment of eosinophils to the lung. Upon sufficient exposure to an allergen, an atopic individual will become sensitized and mount a T helper 2 (Th2) response, which, as explained in more detail later, will lead to the production of allergen-specific IgE antibodies, which in turn will trigger mast cell activation. Upon activation, mast cells produce inflammatory mediators such as histamine, leukotrienes and cytokines, which, along with infiltrating eosinophils, initiate and/or perpetuate most of the pathologies associated with the allergic response. Thus the aim of many treatments for allergic asthma is to prevent or dampen the Th2 immune response and instead promote a Th1 response that results in significantly reduced IgE levels and reduced eosinophilia.
Since the status of antigen-presenting cells (APCs), usually dendritic cells (DCs) or alveolar macrophages, at the time of encounter with the allergen is important with respect to the type of immune response generated, the development of drug-delivery systems to combat allergic asthma has focused on targeting, directly or indirectly, the innate immune system of the lungs [10]. When APCs encounter allergen in atopic individuals they will tend to promote Th2 immune responses [11]. In contrast, when APCs encounter allergen in the presence of certain danger signals, or pathogen-associated molecular patterns (PAMPs), then the induction of Th1 immune responses is more likely. One such danger signal to have garnered the interest of researchers focused on combating allergic asthma is unmethylated CpG-oligonucleotide (CpG-ODN). Although CpG-ODN was implicated as a potential treatment for asthma over 20 years ago, it has yet to reach widespread use [12]. One issue is that, in order to have efficacy, CpG-ODN may often need to be delivered at high doses, which when in a soluble form can result in undesired side-effects such as septic shock-like syndrome caused by an increase in systemic cytokine levels [13]. Several studies have investigated improving the effectiveness of CpG-ODN therapy by using nanoparticles (NPs), which for the purposes of this review are defined as being >10 nm and <1 μm in diameter. Incorporating or conjugating CpG-ODN into or onto NPs (NP-CpG-ODN) engenders a number of potential advantages, which, depending on the NP and the formulation parameters, may include: the protection of CpG-ODN from premature degradation; increasing uptake into cells; prolonged retention at administration sites; improved safety; and providing an opportunity for co-delivery of allergen/antigen and CpG-ODN to the same APC [14–17]. These advantages can culminate in an overall enhanced immunostimulatory potency of NP-CpG-ODN over soluble CpG-ODN. This review, along with describing the rationale behind using CpG-ODN as a potential treatment for allergic asthma, also provides an overview of in vivo studies involving various animal allergy models and clinical trials that have demonstrated the benefits of NP-CpG-ODN in treating allergic asthma.
Immunostimulatory effects of soluble CpG-ODN in allergic asthma
CpG-ODN is a PAMP that binds to its intracellular cognate receptor, TLR-9, which is expressed by all DCs in mice and by a subset of DCs in humans known as plasmacytoid DCs [18]. Additionally, TLR-9 is also expressed by B cells and macrophages in both humans and mice [19]. CpG-ODN can be delineated into separate classes based on functional and structural properties. For humans, three major classes of CpG-ODN have been identified: A-class, B-class and C-class. The details of these classes have been extensively reviewed elsewhere [20,21]. In the subsequent discussion in this review, we will be referring primarily to B-class CpG-ODN unless otherwise specified. B-class CpG-ODN, by binding to TLR-9 in APCs, can trigger a signaling cascade that results in cross-presentation of antigen and the production of Th1 cytokines, such as IL-12, as well as the regulatory cytokine, IL-10 [10].
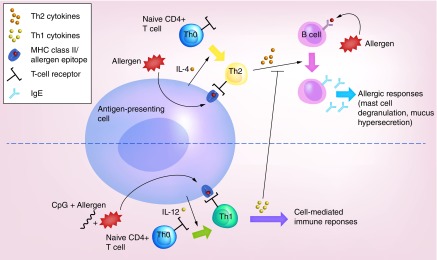
Allergens are presented by APCs, which include alveolar macrophages and DCs, to naive T cells (Th0), which, in atopic individuals, differentiate into Th2 cells [22]. Th2 cells are CD4+ T cells that, upon interaction with an APC-presenting allergen, produce a suite of cytokines that promote IgE production by B cells. In the presence of an appropriate adjuvant, such as CpG-ODN, APCs instead trigger Th1-mediated responses. The release of Th1 cytokines inhibits Th2 cytokine release and therefore abrogates Th2-mediated allergic responses (Figure 1) [23]. Tregs, which are being increasingly recognized as vital players in either suppressing or perpetuating allergic diseases are also affected by CpG-ODN in a complicated dynamic that is yet to be thoroughly understood [24]. Nevertheless, it has been reported that CpG-ODN can not only stimulate Th1 responses but is capable of generating Tregs in both mice and humans, and it has been shown that this can coincide with reduction in symptoms associated with allergic asthma [25,26].
Figure 1. . Schematic depicting the role of CpG-ODN (CpG) in influencing the immune response to an allergen.
The roles of Th1 and Th2 cytokines in allergic asthma, as well as the impact of CpG-ODN treatment, have been investigated using knockout (KO) mice. Several studies using interleukin (IL)-KO mice have confirmed the role of specific ILs in allergic asthma. IL-4 or IL-5 KO C57BL/6 mice sensitized to ovalbumin (OVA) and treated with aerosolized OVA had massively reduced IgE levels or a loss of eosinophilia, respectively, when compared to wild-type mice. It was also observed that AHR responses and other allergy-associated lung damage were abolished in IL-5, but not IL-4 KO mice [27,28]. The findings from these C57BL/6 KO mice were found to differ from studies in BALB/c mice, a more Th2-prone strain, where IL-5 was not deemed important for the induction of AHR [29]. In human clinical trials, however, it has been found that treatment of patients prone to severe eosinophilic asthma with anti-IL-5 antibodies, such as the humanized mepolizumab, caused significant reductions in the risk of onset of asthmatic exacerbations [30]. In addition, a humanized anti-IL-5 receptor antibody, benralizumab, has demonstrated encouraging results in clinical trials and has demonstrated the capacity to cause long-term eradication of blood and airway eosinophils [31]. Further studies, using IL-13 KO mice or IL-13 blocking studies in BALB/c mice, highlighted the role of IL-13 in chronic AHR [32]. It was discovered that IL-13 could act in parallel as well as crosstalk with IL-4 and -5 signaling pathways in order to induce allergic responses [33–35]. These studies confirmed the importance and complexity of Th2 cytokine involvement in the immune response to aeroallergens, but did not investigate the contextual role of Th1 cytokines. KO models of the Th1 cytokines IL-12 and IFN-γ, independently and in concert, were used by Kline et al. to implicate Th2 cells as indirect targets of CpG-ODN [36]. The Th1 cytokine KO C57BL/6 mice were sensitized to Schistosoma mansoni eggs with Schistosoma soluble egg antigen, and both eosinophil counts and serum levels of IL-4 and -5 were reduced in the presence of soluble CpG-ODN [12,36]. Thus, there is some evidence that CpG-ODN therapy can suppress allergic responses independently of the major Th1 cytokines; however, the amounts of CpG-ODN required to achieve comparable blocking of Th2 responses to that shown in wild-type mice were found to be an order of magnitude greater [36]. Additionally, in a separate study, CpG-ODN treatment of IFN-γ KO BALB/c mice sensitized to ragweed was ineffective at reducing eosinophils in the airways, which was in contrast to when wild-type mice were used [37]. These findings suggest multiple mechanisms by which CpG-ODN can suppress Th2 responses where at least one mode was independent of IFN-γ and IL-12. Although not investigated by Kline et al., subsequent independent studies in different allergy models suggested a role for IL-10 in CpG-ODN-mediated suppression of allergic Th2 responses [38]. Another potential mediator of CpG-ODN-induced suppression is the more recently discovered and enigmatic cytokine, IL-22, which can be upregulated in expression in DCs upon stimulation with CpG-ODN [39]. In separate studies, IL-22 has been shown to both promote and abrogate symptoms of allergic asthma, and it has been suggested that these apparently paradoxical roles may depend on the presence or absence of IL-17, respectively [40]. A summary of the cytokines mentioned in this review, and their role in allergic responses is shown in Table 1 [41,42].
Table 1. . List of cytokines referenced in this review article, the T-cell lineage to which they belong, and their role(s) in allergic responses.
| Cytokine | T lineage | Elicited response |
|---|---|---|
| IFN-γ | Th1 | Inhibits IgE synthesis, inhibits Th2 induction, indirectly inhibits eosinophil recruitment to the lung, stimulates IL-12 production by APCs |
| IL-4 | Th2 | B-cell isotype switch to IgE production, activates eosinophils and basophils, increases mucus production and secretion |
| IL-5 | Th2 | Recruits and activates eosinophils, stimulates basophil differentiation, maturation and activation |
| IL-6 | Th1/Th2 | Growth factor for T and B cells, co-factor for IgE synthesis |
| IL-10 | Treg | Suppresses both Th1 and Th2 functions, activates eosinophils and basophils, B cell switch to IgG4 expression |
| IL-12 | Th1 | Inhibits IgE synthesis, promotes IFN-γ production |
| IL-13 | Th2 | Increases mucus production, B cell switch to IgE production, recruits eosinophils, promotes airway hyperresponsiveness |
| TNF-α | Th1 | May contribute to the pathogenesis of severe allergic asthma |
| IL-17 | Th17 | Proinflammatory/increases severity of allergic asthma |
| IL-22 | Th17 | Ambiguous role (can suppress and promote asthmatic symptoms) |
| TSLP | – | Promotes Th2 allergic asthma responses/Th2 memory |
In vivo models for treating allergic asthma with CpG-ODN include therapeutic treatment, in which CpG-ODN therapy is administered after animals have been sensitized to a particular allergen. Conversely, prophylactic treatment occurs when CpG-ODN is administered prior to allergen sensitization in animals. Co-delivery of CpG-ODN and the allergen is also conducted as a type of prophylactic treatment. In each model, CpG-ODN and/or the allergen are delivered by a variety of routes including intranasal instillation, subcutaneous, intravenous or intraperitoneal injections.
Soluble CpG-ODN delivered to allergic or preallergic mice has been shown to reduce hallmark responses of allergic asthma when compared with untreated mice. For example, the effects of intranasally delivered CpG-ODN to BALB/c mice 3 days prior to a sensitization regime with cockroach allergen resulted in reductions in AHR, eosinophils in the lavage fluid, total IgE, cockroach-specific IgE, IL-13 and IL-5, compared with mice pretreated with a nonimmunostimulatory ODN. In addition, it was found that IFN-γ levels increased while IL-10 levels exhibited no change [43]. Similar results were obtained when mice were simultaneously treated with cockroach allergen and CpG-ODN as well as when mice were given CpG-ODN immediately after the allergen sensitization regime.
Cockroach and house dust mite (HDM) allergies affect up to 40 and 60% of American individuals, respectively [44]. In addition, allergic rhinitis and asthma often co-exist in what is known as combined allergic rhinitis and asthma syndrome (CARAS), thus emphasizing the inter relationship between the lower and upper airways [45]. In a study involving a CARAS model, BALB/c mice were sensitized to OVA intraperitoneally and subsequently challenged with CpG-ODN and OVA intranasally which was followed by OVA aerosol exposure (lower airway) in an attempt to establish that delivery of CpG-ODN to the upper airway could affect lower airway inflammation [46]. It was found that upper airway treatment of CARAS mice with soluble CpG-ODN significantly reduced the symptoms of allergic rhinitis, lower airway inflammation and AHR. Intradermal administration of soluble CpG-ODN, on the other hand, was ineffective. The same group subsequently investigated the mechanism by which CpG-ODN may have mediated its effects in the CARAS mice and concluded that CpG-ODN may potentially suppress the activity of TSLP, an epithelial/fibroblastic-derived protein that is known to promote pulmonary Th2 responses to allergen [46,47]. Allergies caused by Japanese cedar pollen (JCP) affect approximately 10% of the Japanese population annually, and is therefore an important model for consideration in allergic asthma treatment. In a prophylactic study, BALB/c mice were treated intraperitoneally with CpG-ODN 14 days prior to intranasal sensitization with JCP [48]. Mice pretreated with CpG-ODN had significantly decreased IgE levels, decreased pulmonary infiltration of eosinophils, as well as reduced secretion of IL-4, -5 and -17 in the lungs compared with JCP-sensitized mice receiving no CpG-ODN pretreatment. Therefore, prophylactic treatment with CpG-ODN is a promising approach to abating the inflammatory consequences of JCP exposure in the Japanese population. Together, these studies suggest that soluble CpG-ODN is a potentially beneficial agent in the treatment of allergic asthma, which begs the question as to whether the efficacy of this PAMP can be further improved by nanoparticularization.
NP-CpG-ODN treatment of allergic asthma with & without allergen
NPs can be delivered to the lungs through inhalation, thus having a direct effect on the lung microenvironment. While delivery of NPs to the lungs can hold many advantages over delivery through other routes, there are often problematic issues that need to be overcome. Advantages include the high absorption area of the lungs and the noninvasiveness of the delivery method. Disadvantages include potential for NP agglomeration and low efficiency of delivery due to exhalation or mucus- and cilia-assisted expulsion. Until recently NP delivery to the lung has mostly been in the context of delivery of drugs such as insulin and antibiotics where one of the priorities is that the NPs avoid being phagocytosed and therefore prolong the presence of the NP for sustained drug release. This, for the most part, is in contrast to NP delivery for the purposes of combating allergic asthma, where the primary target is often phagocytic APCs such as DCs and alveolar macrophages [49]. Although promising results have been obtained in the aforementioned studies using soluble CpG-ODN, the advantages of utilizing NP-CpG-ODN instead have been noted in preclinical allergic asthma models. NPs loaded or conjugated to CpG-ODN offer a number of advantages over soluble CpG-ODN treatment including enhanced targeting, potential for co-delivery applications (e.g., allergen plus adjuvant) to APCs, enhanced protection and sufficient residence time, as well as reduction of local and systemic cytotoxicity [50]. Ultimately, the main advantage that NPs offer is their potential for generating vaccines/treatments that are both safer and more effective than soluble formulations. For example, in a prophylactic study involving NPs made from polypropylene sulfide, C57BL/6 mice were pretreated, intranasally (days 1, 3 and 5), with 2 μg soluble CpG-ODN or NP-CpG-ODN (30 nm diameter), sensitized to HDM (days 8, 10 and 12) and then challenged with HDM (days 22, 24 and 26), while in a therapeutic study by the same group, mice were sensitized with HDM (days 1, 3 and 5), treated with 2 μg instillations of soluble CpG-ODN or NP-CpG-ODN (days 15, 17 and 19) and then challenged with HDM (days 22, 24 and 26) [51]. In the prophylactic study NP-CpG-ODN-treated mice had reduced lung eosinophilia, lower levels of IL-4 and -5 in the lungs and reduced mucus production compared with untreated mice and mice receiving soluble CpG-ODN. In the therapeutic study, both soluble and NP-CpG-ODN were capable of reducing eosinophilia to a similar extent; however, NP-CpG-ODN could significantly reduce IL-5, IL-13 and TGF-β1 in the lungs compared with soluble CpG-ODN. This study also highlights the possibility that smaller doses of CpG-ODN can be delivered when used in particle form, thus minimizing the potential for CpG-ODN-induced toxicities. In order for soluble CpG-ODN to achieve similar abrogation of allergic symptoms, it has been found in separate studies in mice that doses of 10–100 μg are required [10,52]. These doses of soluble CpG-ODN may increase neutrophil and macrophage counts in murine airways after intratracheal exposure [53].
In the above study, pluronic-stabilized poly(propylene sulfide) NPs were synthesized, then functionalized with pyridyl disulfide and then surface-conjugated to CpG-ODN [51,54]. Part of the rationale behind using these NPs was that, being ultra small (30 nm), they could independently drain to lymph nodes and therefore be taken up by resident lymph node DCs, known to be potent APCs [55]. Another type of ultra small NP-CpG-ODN-based allergy treatment involves QbG10, a 30 nm nonreplicating virus-like particle derived from the bacteriophage Qβ, encapsulating G10, an A-type CpG-ODN. This type of CpG-ODN, unlike B-type CpG-ODN, tends to promote IFN-α, rather than IL-12, secretion by plasmacytoid DCs, which in humans (but not mice) results in dampening of Th2 responses [56]. The NP was formed by packaging G10 into the virus-like particle during self assembly [57]. A recent parallel randomized human clinical trial was performed with patients with mild to moderate persistent allergic asthma where 33 patients were treated with seven subcutaneous injections of 0.9 mg QbG10 and 30 patients received a placebo [58]. The study concluded that QbG10 had a significant positive effect on asthma control with improved patient-reported parameters as well as stable FEV1 (forced expiratory volume) and significantly reduced eosinophils in the blood. The mechanism by which QbG10 exerted its effects was not investigated; however, the authors suggest that the observed improvements were likely mediated by plasmacytoid DCs which readily take up QbG10 NPs and respond to TLR-9 agonists by producing type 1 interferons, which in turn dampen Th2 responses [56,58].
Thus far, the discussed NP-CpG-ODN studies have been used as stand-alone (or allergen-free) treatments. Many of the NP formulations have the potential to co-deliver an allergen with CpG-ODN for the purpose of allergen-specific immunotherapy. Most NPs are amenable to surface attachment of CpG-ODN or allergen by chemical conjugation. In addition, NPs can be loaded with CpG-ODN, as described above for QbG10 and then subsequently surface modified with the allergen. In the case of NPs made from the biodegradable polymer poly(lactic-co-glycolic) acid both agents can be readily co-loaded (or encapsulated) or alternatively one agent can be encapsulated while the other absorbed or chemically linked to the surface. Our group recently manufactured 300 nm poly(lactic-co-glycolic) acid NPs loaded with CpG-ODN and surface adsorbed to the common HDM (Dermatophagoides pteronyssinus) allergen, Derp2, for use as subcutaneous vaccines in C3H/HeBFeJ mice that were subsequently sensitized to Derp2 [59]. Derp2 is known to produce strong immunogenic responses [60]. The presence of CpG-ODN in the formulation improved the Th1 response and reduced perivascular cuffing as well as lowered the number of pulmonary eosinophils compared with vaccines with NPs delivering Derp2 only [59].
There are a number of other NP-based formulations incorporating CpG-ODN that have shown promise in in vitro and, in some cases, in vivo studies but are yet to be tested in in vivo models for allergic asthma. Table 2 summarizes those CpG-NPs that have been used in allergic asthma models (described above) as well as these promising but yet untested NPs. These include self-assembled CpG-ODN-containing DNA nanostructures, AuNPs and chitosan-coated boron nitride nanospheres. CpG-ODN polypodna are self-assembling nanostructures of approximately 6–12 nm in diameter comprising ODNs that include CpG-ODN motifs. Another type of DNA nanostructure being investigated as an immunostimulatory adjuvant includes self-assembled DNA tetrahedra appended with CpG-ODN motifs. Both these types of nanostructures were shown to stimulate the expression of IL-6, TNF-α and/or IL-12 by the murine macrophage cell line (RAW264.7) in vitro [61,62]. More recently, it was shown that polypod-like DNA nanostructures incorporating CpG-ODN could also stimulate IL-12 production in vivo to levels 20-fold greater than soluble CpG-ODN upon intravenous administration to mice [63]. In a separate study it was shown that self-assembled CpG-ODN-conjugated AuNPs (15 and 30 nm diameters) could be taken up more efficiently by RAW264.7 cells as well as enhance the secretion of IL-6, TNF-α and IL-10, when compared with soluble CpG-ODN [64]. Finally, chitosan-coated boron nitride nanospheres surface-loaded with CpG-ODN were shown to significantly enhance TNF-α and IL-6 secretion by human peripheral blood mononuclear cells in vitro compared with soluble CpG-ODN [65]. Whether or not any of these novel CpG-ODN-NPs have any potential as therapies for allergic asthma remains to be elucidated through preclinical allergic asthma animal model studies and/or clinical trials. A summary of the aforementioned studies performed with these various NP-CpG-ODN formulations is presented in Table 2.
Table 2. . Summary of nanoparticle-CpG-ODN formulations used in in vivo and in vitro experiments and results of each study.
| NP-CpG-ODN formulation | NP size (nm) | Experimental/clinical study | Key result(s) |
|---|---|---|---|
| Poly(propylene sulfide) NPs surface conjugated to CpG-ODN | 30 | Prophylactic and therapeutic HDM model (mouse/in vivo) (versus soluble CpG-ODN) [51] | ↓eosinophilia, IL-5, IL-4 (prophylactic). ↓IL-5, IL-13 and TGF-β1 (therapeutic) |
| QbG10 (VLPs encapsulating A-type CpG-ODN) | 30 | Human clinical trial (versus placebo) [58] | ↓eosinophils in blood stable FEV1 |
| Poly(lactic-co-glycolic) acid NPs co-loaded with Derp2 and CpG-ODN | 300 | Specific antigen vaccination in HDM model (mouse/in vivo) (versus NPs without CpG-ODN) [59] | ↑Th1 responses. ↓Perivascular cuffing |
| CpG-ODN polypodna | 6–12 | Intravenous administration (mice/in vivo)(versus soluble CpG-ODN) [63] | ↑ IL-12 in plasma |
| AuNPs conjugated to CpG-ODN | 15 and 30 | Uptake by, and stimulation of, RAW264.7 cells (in vitro) (versus soluble CpG-ODN) [64] | ↑ TNF-α, IL-6 and IL-10 secretion |
| Chitosan-coated BNNS surface loaded with CpG-ODN | 400–500 | Stimulation of human PBMNCs (in vitro) (versus soluble CpG-ODN) [65] | ↑ TNF-α and IL-6 secretion |
BNNS: Boron nitride nanospheres; FEV1: Forced expiratory volume; HDM: House dust mite; NP: Nanoparticle; ODN: Oligonucleotide; PBMNC: Peripheral blood mononuclear cell; VLP: Virus-like particle.
Conclusion
CpG-ODN has been shown to abrogate Th2 responses and diminish the symptoms associated with allergic asthma. The mechanisms by which it does so include promoting Th1 responses, upregulating IL-10 production and inhibiting TSLP. Formulating NP-CpG-ODNs appears to be a promising way forward in terms of long-term abatement of this disease. Evidence thus far from the few preclinical animal experiments and clinical studies performed demonstrates a superiority of NP-CpG-ODN over the soluble form in terms of required delivery dose, abatement of allergic symptoms and their causes (such as Th2 cytokines and eosinophilia). While there are lessons to be learned from previous studies investigating pulmonary NP drug delivery for other diseases (e.g., diabetes), this must be tempered by the knowledge that NP-CpG-ODN therapy generally requires the physical engagement and uptake by APCs. Thus, rather than avoiding phagocytosis, NPs designed to target APCs are instead required to be phagocytosed. Therefore, the size, mucoadhesive and penetrating properties of the NPs are important for optimal pulmonary delivery. When co-delivering allergen and CpG-ODN as a vaccine, NPs have the potential to promote allergen-specific immunotherapy more efficiently than soluble combinations. It remains to be seen whether allergen-specific or allergen-free CpG-ODN-NPs will be the preferable options in the path toward allergic asthma therapy.
Future perspective
Allergic asthma is a common problem in the developed world, and many of the common aeroallergens are abundant in the environment and virtually impossible to avoid. Thus, developing a treatment for these allergies is currently a critical need. Studies regarding comparisons of NP aerodynamic and geometric size effects and mucoadhesive and penetrating properties will be a boon to the field because these are the critical gaps in developing safe and effective treatment for the disease. NP-CpG-ODNs offer an opportunity to replace current conventional treatments, which only temporarily abate the asthmatic symptoms, by instead providing an immune-based approach that provides long-term protection against allergic asthma. Ideally, the development of a readily patient compliant formulation, which is delivered by inhalation, can be stably stored, provides long-term relief and little or no side effects is a desirable goal. To reach this goal further optimization of formulations and preclinical and clinical studies need to be performed.
Executive summary.
Section 1: current conventional treatments for allergic asthma do not attack the cause of the disease and offer only temporary abatement from symptoms. Nanoparticle-CpG-oligonucleotides (ODN) provide the potential for long-term abatement of allergic asthma by efficiently suppressing T helper 2 (Th2) responses.
Section 2: soluble CpG-ODN can suppress Th2-driven allergic immune responses in animal asthma models via a variety of not necessarily mutually exclusive mechanisms that include promotion of Th1 cytokines and IL-10 by antigen-presenting cells and suppression of TSLP.
Section 3: delivery of CpG-oligonucleotides in nanoparticle form has demonstrated improved efficiency over soluble CpG-ODN in suppressing the symptoms of allergic asthma as determined from preclinical and clinical studies.
Footnotes
Financial & competing interests disclosure
Brittany E Givens acknowledges fellowship support from the Alfred P. Sloan Foundation, the University of Iowa Graduate College and the National GEM Consortium. Aliasger K Salem acknowledges support from NIH P30 ES005605, U01ES027252 01, and the Lyle and Sharon Bighley Chair of Pharmaceutical Sciences. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.Morris AS, Sebag SC, Paschke JD, et al. Cationic CaMKII inhibiting nanoparticles prevent allergic asthma. Mol. Pharm. 2017;14(6):2166–2175. doi: 10.1021/acs.molpharmaceut.7b00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pakhale S, Mulpuru S, Boyd M. Optimal management of severe/refractory asthma. Clin. Med. Insights. Circ. Respir. Pulm. Med. 2011;5:37–47. doi: 10.4137/CCRPM.S5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penttinen P, Timonen KL, Tiittanen P, Mirme A, Ruuskanen J, Pekkanen J. Ultrafine particles in urban air and respiratory health among adult asthmatics. Eur. Respir. J. 2001;17(3):428–435. doi: 10.1183/09031936.01.17304280. [DOI] [PubMed] [Google Scholar]
- 4.Schatz M, Rosenwasser L. The allergic asthma phenotype. J. Allergy Clin. Immunol. Pract. 2014;2(6):645–648. doi: 10.1016/j.jaip.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Ober C, Leavitt SA, Tsalenko A, et al. Variation in the interleukin 4-receptor α gene confers susceptibility to asthma and atopy in ethnically diverse populations. Am. J. Hum. Genet. 2000;66(2):517–526. doi: 10.1086/302781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devereux G. The increase in the prevalence of asthma and allergy: food for thought. Nat. Rev. Immunol. 2006;6(11):869–874. doi: 10.1038/nri1958. [DOI] [PubMed] [Google Scholar]; • Highlights the increasing prevalence of allergic asthma in the western world and discusses the hypothetical role of diet.
- 7.Erle DJ, Sheppard D. The cell biology of asthma. J. Cell Biol. 2014;205(5):621–631. doi: 10.1083/jcb.201401050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta GK, Agrawal DK. CpG oligodeoxynucleotides as TLR9 agonists therapeutic application in allergy and asthma. BioDrugs. 2010;24(4):225–235. doi: 10.2165/11536140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 10.Fonseca DE, Kline JN. Use of CpG oligonucleotides in treatment of asthma and allergic disease. Adv. Drug Deliv. Rev. 2009;61(3):256–262. doi: 10.1016/j.addr.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Fahy JV. Type 2 inflammation in asthma – present in most, absent in many. Nat. Rev. Immunol. 2015;15:57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kline JN, Waldschmidt TJ, Businga TR, et al. Cutting edge: modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J. Immunol. 1998;160(6):2555–2559. [PubMed] [Google Scholar]
- 13.Von Beust BR, Johansen P, Smith KA, Bot A, Storni T, Kundig TM. Improving the therapeutic index of CpG oligodeoxynucleotides by intralymphatic administration. Eur. J. Immunol. 2005;35(6):1869–1876. doi: 10.1002/eji.200526124. [DOI] [PubMed] [Google Scholar]
- 14.Krishnamachari Y, Salem AK. Innovative strategies for co-delivering antigens and CpG oligonucleotides. Adv. Drug Deliv. Rev. 2009;61(3):205–217. doi: 10.1016/j.addr.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kachura MA, Hickle C, Kell SA, et al. A CpG-Ficoll nanoparticle adjuvant for anthrax protective antigen enhances immunogenicity and provides single-immunization protection against inhaled anthrax in monkeys. J. Immunol. 2016;196(1):284–297. doi: 10.4049/jimmunol.1501903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Titta A, Ballester M, Julier Z, et al. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc. Natl Acad. Sci. USA. 2013;110(49):19902–19907. doi: 10.1073/pnas.1313152110. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This article emphasizes the advantages of conjugating CpG to ultrasmall nanoparticles in terms of targeting lymph nodes, enhanced cellular immunity and efficient dosing.
- 17.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010;10(11):787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 18.Miles SA, Sandler AD. CpG oligonucleotides for immunotherapeutic treatment of neuroblastoma. Adv. Drug Deliv. Rev. 2009;61(3):275–282. doi: 10.1016/j.addr.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 20.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006;(6):471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 21.Vollmer J, Weeratna R, Payette P, et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur. J. Immunol. 2004;34(1):251–262. doi: 10.1002/eji.200324032. [DOI] [PubMed] [Google Scholar]
- 22.Klimek L, Bachmann MF, Senti G, Kundig TM. Immunotherapy of type-1 allergies with virus-like particles and CpG-motifs. Expert Rev. Clin. Immunol. 2014;10(8):1059–1067. doi: 10.1586/1744666X.2014.924854. [DOI] [PubMed] [Google Scholar]
- 23.Holt PG. A potential vaccine strategy for asthma and allied atopic diseases during early childhood. Lancet. 1994;344(8920):456–458. doi: 10.1016/s0140-6736(94)91776-0. [DOI] [PubMed] [Google Scholar]
- 24.Noval Rivas M, Chatila TA. Regulatory T cells in allergic diseases. J. Allergy Clin. Immunol. 2016;138(3):639–652. doi: 10.1016/j.jaci.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohammadi-Shahrokhi V, Rezaei A, Andalib A, Rahnama A, Jafarzadeh A, Eskandari N. Immunomodulatory effects of adjuvants CPG, MPLA, and BCG on the Derp2-induced acute asthma at early life in an animal model of BALB/c mice. Inflammation. 2017;40(1):259–274. doi: 10.1007/s10753-016-0476-2. [DOI] [PubMed] [Google Scholar]
- 26.Moseman EA, Liang X, Dawson AJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. 2004;173(7):4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 27.Brusselle G, Kips J, Joos G, Bluethmann H, Pauwels R. Allergen-induced airway inflammation and bronchial responsiveness in wild-type and interleukin-4-deficient mice. Am. J. Respir. Cell Mol. Biol. 1995;12(3):254–259. doi: 10.1165/ajrcmb.12.3.7873190. [DOI] [PubMed] [Google Scholar]
- 28.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J. Exp. Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Salient study using IL-5 KO mice demonstrating the importance of IL-5 and eosiniphils in mediating allergic asthma.
- 29.Corry DB, Folkesson HG, Warnock ML, et al. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J. Exp. Med. 1996;183(1):109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 31.Tan LD, Bratt JM, Godor D, Louie S, Kenyon NJ. Benralizumab: a unique IL-5 inhibitor for severe asthma. J. Asthma Allergy. 2016;9:71–81. doi: 10.2147/JAA.S78049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hogan SP, Matthaei KI, Young JM, Koskinen A, Young IG, Foster PS. A novel T cell-regulated mechanism modulating allergen-induced airways hyperreactivity in BALB/c mice independently of IL-4 and IL-5. J. Immunol. 1998;161(3):1501–1509. [PubMed] [Google Scholar]
- 33.Webb DC, Mckenzie ANJ, Koskinen AML, Yang M, Mattes J, Foster PS. Integrated signals between IL-13, IL-4, and IL-5 regulate airways hyperreactivity. J. Immunol. 2000;165(1):108–113. doi: 10.4049/jimmunol.165.1.108. [DOI] [PubMed] [Google Scholar]
- 34.Jain VV, Businga TR, Kitagaki K, George CL, O'Shaughnessy PT, Kline JN. Mucosal immunotherapy with CpG oligodeoxynucleotides reverses a murine model of chronic asthma induced by repeated antigen exposure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285(5):L1137–1146. doi: 10.1152/ajplung.00073.2003. [DOI] [PubMed] [Google Scholar]
- 35.Taube C, Duez C, Cui ZH, et al. The role of IL-13 in established allergic airway disease. J. Immunol. 2002;169(11):6482–6489. doi: 10.4049/jimmunol.169.11.6482. [DOI] [PubMed] [Google Scholar]
- 36.Kline JN, Krieg AM, Waldschmidt TJ, Ballas ZK, Jain V, Businga TR. CpG oligodeoxynucleotides do not require T-H1 cytokines to prevent eosinophilic airway inflammation in a murine model of asthma. J. Allergy Clin. Immunol. 1999;104(6):1258–1264. doi: 10.1016/s0091-6749(99)70022-9. [DOI] [PubMed] [Google Scholar]
- 37.Sur S, Wild JS, Choudhury BK, Sur N, Alam R, Klinman DM. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. J. Immunol. 1999;162(10):6284–6293. [PubMed] [Google Scholar]
- 38.Kitagaki K, Jain VV, Businga TR, Hussain I, Kline JN. Immunomodulatory effects of CpG oligodeoxynucleotides on established Th2 responses. Clin. Vaccine Immunol. 2002;9(6):1260–1269. doi: 10.1128/CDLI.9.6.1260-1269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fumagalli S, Torri A, Papagna A, Citterio S, Mainoldi F, Foti M. IL-22 is rapidly induced by pathogen recognition receptors stimulation in bone-marrow-derived dendritic cells in the absence of IL-23. Sci. Rep. 2016;6:33900. doi: 10.1038/srep33900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J. Exp. Med. 2010;207(6):1293–1305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holgate ST, Dent G, Buckley MG. Asthma. In: Runge MS, Patterson C, editors. Principles of Molecular Medicine. Humana Press, Inc.; Tokowa, NJ, USA: 2006. pp. 198–213. [Google Scholar]
- 42.Larché M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J. Allergy Clin. Immunol. 2003;111(3):450–463. doi: 10.1067/mai.2003.169. [DOI] [PubMed] [Google Scholar]
- 43.Kim DH, Sohn JH, Park HJ, Lee JH, Park JW, Choi JM. CpG oligodeoxynucleotide Inhibits cockroach-induced asthma via induction of IFN-γ(+) Th1 cells or Foxp3(+) regulatory T cells in the lung. Allergy Asthma Immunol. Res. 2016;8(3):264–275. doi: 10.4168/aair.2016.8.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peden D, Reed CE. Environmental and occupational allergies. J. Allergy Clin. Immunol. 2010;125(2 Suppl. 2):S150–160. doi: 10.1016/j.jaci.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 45.Taramarcaz P, Gibson PG. The effectiveness of intranasal corticosteroids in combined allergic rhinitis and asthma syndrome. Clin. Exp. Allergy. 2004;34(12):1883–1889. doi: 10.1111/j.1365-2222.2004.02130.x. [DOI] [PubMed] [Google Scholar]
- 46.Li HT, Zhang TT, Chen ZG, et al. Intranasal administration of CpG oligodeoxynucleotides reduces lower airway inflammation in a murine model of combined allergic rhinitis and asthma syndrome. Int. Immunopharmacol. 2015;28(1):390–398. doi: 10.1016/j.intimp.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 47.Li HT, Chen ZG, Liu H, et al. Treatment of allergic rhinitis with CpG oligodeoxynucleotides alleviates the lower airway outcomes of combined allergic rhinitis and asthma syndrome via a mechanism that possibly involves in TSLP. Exp. Lung Res. 2016;42(6):322–333. doi: 10.1080/01902148.2016.1215571. [DOI] [PubMed] [Google Scholar]
- 48.Liu B, Zhang L, Liu J, Shan F, Wang E, Kimura Y. A prophylactic effect of an oligodeoxynucleotide containing a cytidine-guanosine motif against Japanese cedar pollen-induced T-helper type 2 allergic response. J. Asthma. 2011;48(9):974–978. doi: 10.3109/02770903.2011.619288. [DOI] [PubMed] [Google Scholar]
- 49.Blank F, Fytianos K, Seydoux E, et al. Interaction of biomedical nanoparticles with the pulmonary immune system. J. Nanobiotechnol. 2017;15(1):6. doi: 10.1186/s12951-016-0242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanagata N. Structure-dependent immunostimulatory effect of CpG oligodeoxynucleotides and their delivery system. Int. J. Nanomed. 2012;7:2181–2195. doi: 10.2147/IJN.S30197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ballester M, Jeanbart L, De Titta A, et al. Nanoparticle conjugation enhances the immunomodulatory effects of intranasally delivered CpG in house dust mite-allergic mice. Sci. Rep. 2015;5:14274. doi: 10.1038/srep14274. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates the advantage of pulmonary delivery of nanoparticle-conjugated CpG over soluble CpG in reducing allergic responses to HDM allergen in both therapeutic and prophylactic settings.
- 52.Campbell JD, Kell SA, Kozy HM, et al. A limited CpG-containing oligodeoxynucleotide therapy regimen induces sustained suppression of allergic airway inflammation in mice. Thorax. 2014;69(6):565–573. doi: 10.1136/thoraxjnl-2013-204605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duechs MJ, Hahn C, Benediktus E, et al. TLR agonist mediated suppression of allergic responses is associated with increased innate inflammation in the airways. Pulm. Pharmacol. Ther. 2011;24(2):203–214. doi: 10.1016/j.pupt.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 54.Van Der Vlies AJ, O'neil CPO, Hasegawa U, Hammond N, Hubbell JA. Synthesis of pyridyl disulfide-functionalized nanoparticles for conjugting thiol-containing small molecules, pepties, and proteins. Bioconjugate Chem. 2010;21:653–662. doi: 10.1021/bc9004443. [DOI] [PubMed] [Google Scholar]
- 55.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Rel. 2006;112(1):26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 56.Huber JP, Ramos HJ, Gill MA, Farrar JD. Cutting edge: type I IFN reverses human Th2 commitment and stability by suppressing GATA3. J. Immunol. 2010;185(2):813–817. doi: 10.4049/jimmunol.1000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storni T, Ruedl C, Schwarz K, Schwendener RA, Renner WA, Bachmann MF. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J. Immunol. 2004;172(3):1777–1785. doi: 10.4049/jimmunol.172.3.1777. [DOI] [PubMed] [Google Scholar]
- 58.Beeh KM, Kanniess F, Wagner F, et al. The novel TLR-9 agonist QbG10 shows clinical efficacy in persistent allergic asthma. J. Allergy Clin. Immunol. 2013;131(3):866–874. doi: 10.1016/j.jaci.2012.12.1561. [DOI] [PubMed] [Google Scholar]
- 59.Joshi VB, Adamcakova-Dodd A, Jing X, et al. Development of a poly (lactic-co-glycolic acid) particle vaccine to protect against house dust mite induced allergy. AAPS J. 2014;16(5):975–985. doi: 10.1208/s12248-014-9624-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kidon MI, Chiang WC, Liew WK, et al. Mite component-specific IgE repertoire and phenotypes of allergic disease in childhood: the tropical perspective. Pediatr. Allergy Immunol. 2011;22(2):202–210. doi: 10.1111/j.1399-3038.2010.01094.x. [DOI] [PubMed] [Google Scholar]
- 61.Mohri K, Nishikawa M, Takahashi N, et al. Design and development of nanosized DNA assemblies in polypod-like structures as efficient vehicles for immunostimulatory CpG motifs to immune cells. ACS Nano. 2012;6(7):5931–5940. doi: 10.1021/nn300727j. [DOI] [PubMed] [Google Scholar]
- 62.Li J, Pei H, Zhu B, et al. Self-assembled multivalent DNA nanostructures for noninvasive intracellular delivery of immunostimulatory CpG oligonucleotides. ACS Nano. 2011;5(11):8783–8789. doi: 10.1021/nn202774x. [DOI] [PubMed] [Google Scholar]
- 63.Takahashi Y, Maezawa T, Araie Y, Takahashi Y, Takakura Y, Nishikawa M. In vitro and in vivo stimulation of Toll-like receptor 9 by CpG oligodeoxynucleotides incorporated into polypod-like DNA nanostructures. J. Pharm. Sci. 2017;106(9):2457–2462. doi: 10.1016/j.xphs.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 64.Wei M, Chen N, Li J, et al. Polyvalent immunostimulatory nanoagents with self-assembled CpG oligonucleotide-conjugated gold nanoparticles. Angew. Chem. Int. Ed. 2012;51(5):1202–1206. doi: 10.1002/anie.201105187. [DOI] [PubMed] [Google Scholar]
- 65.Zhang H, Chen S, Zhi C, Yamazaki T, Hanagata N. Chitosan-coated boron nitride nanospheres enhance delivery of CpG oligodeoxynucleotides and induction of cytokines. Int. J. Nanomedicine. 2013;8:1783–1793. doi: 10.2147/IJN.S43251. [DOI] [PMC free article] [PubMed] [Google Scholar]