Abstract
Over the past decade, there has been a rise in the number of clinical cases of moderate to severe anterior segment ocular diseases. Conventional topical ophthalmic formulations have several limitations – to address which, novel drug-delivery systems are needed. Additionally, formidable physiological barriers limit ocular bioavailability through the topical route of application. During the last decade, various nano-scaled ocular drug-delivery strategies have been reported. Some of these exploratory, topical, noninvasive approaches have shown promise in improving penetration into the anterior segment tissues of the eye. In this article, we review the available literature with respect to the safety, efficiency and effectiveness of these nano systems.
Keywords: : anterior segment, nanoparticles, noninvasive, topical
Ocular drug delivery has remained a challenging task for pharmaceutical scientists. This, coupled with the aging population and manifestation of other age-related diseases, explains why the National Eye Institute has predicted a significant rise in clinical cases involving diseases affecting the ocular segments, across various age and ethnic groups [1]. This review focuses on the rising use of noninvasive nanotechnology to improve the therapeutic outcomes in diseases affecting the anterior segment of the eye. The frequently encountered sight-threatening anterior segment ocular diseases are cataract, glaucoma, keratitis, ocular hypertension and uveitis [2–5]. According to the National Eye Institute and the WHO, cataract is one of the major causes of blindness, accounting for 51% of the blindness worldwide [1,6–8].
Topical administration, a localized noninvasive technique, is the most preferred route of dosing for anterior segment ocular diseases. The topical route, however, is associated with low ocular bioavailability (<5% of the administered dose) because of various physiological and mechanical barriers [5]. Conventional topical ophthalmic formulations include eye drops (solutions, suspensions) and ointments. Some emulsion formulations are also currently in the market (Restasis®, Cationorm®). Limited retention on the ocular surface and the need for the drug candidate to possess ideal physicochemical characteristics, to facilitate efficient penetration through the complex ocular structures, however, limits the efficiency of these dosage forms [9]. Solutions are rapidly drained from the conjunctival cul-de-sac allowing very little time for the drug to partition into the ocular tissues. Moreover, the instilled drop size must be in the range of 25–50 μl, which does not allow for the development of a high concentration gradient across the ocular tissues and demands good solubility characteristics, especially at the pH of the tear fluids.
To improve the efficacy of the topical ophthalmic solutions or suspensions, high viscosity (thixotropic or shear thinning) solutions and hydrogels, have been developed to increase retention on the ocular surface [10]. Moreover, in contrast to solutions, suspension formulations are better retained on the ocular surface because of the deposition of the drug particles in the conjunctival cul-de-sac. Suspensions are also the primary choice for compounds with poor aqueous solubility (in relation to the target dose). Compared with solutions and suspensions, ointments are not commonly used for conditions affecting the eye because of the oily components that affect vision for some time following application. In all cases, drainage through the nasolacrimal duct and systemic absorption, and consequent side effects must be carefully monitored.
The conventional formulation approaches discussed above can only increase the retention time on the ocular surface and do not improve transmembrane penetration (in the absence of penetration enhancing formulation components). The complex ultrastructure of the cornea, enzymes, efflux proteins and the lymphatic and vascular systems, severely limits penetration of the therapeutic agent across the ocular tissues. Moreover, the ocular tissues are very sensitive; thus, the use of permeation enhancers is limited in ophthalmic formulations [11,12]. Therefore, there is a pressing need for advanced ophthalmic formulations to circumvent the challenges associated with topical ocular drug delivery.
Prior to looking at the potential formulation strategies, a brief discussion with respect to the diffusion pathways and the diffusional barriers encountered in topical administration is presented.
Routes of permeation following topical application
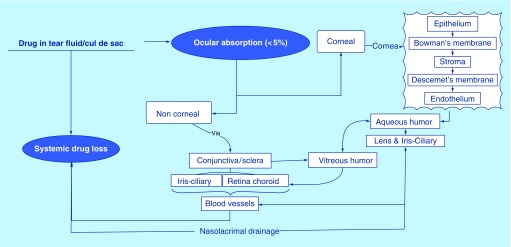
Topically administered drugs can penetrate the deeper ocular tissues primarily through two routes (Figure 1). Transcorneal permeation – commonly referred to as the corneal pathway – is the predominant diffusional route from the ocular surface into the deeper ocular tissues, especially for access to the anterior segment tissues [13]. However, for efficient transcorneal penetration, the physicochemical characteristics of the drug must be favorable and a delicate balance between hydrophilic and lipophilic characteristics is needed. Hydrophilic compounds appear to favor the noncorneal pathway, which involves permeation across the conjunctiva and sclera (conjunctival–scleral pathway) into the deeper tissues of the eye since the conjunctival epithelium is leakier than the corneal epithelium. The conjunctival–scleral pathway is especially attractive for targeting the posterior segment tissues. The conjunctival and choroidal vasculature and lymphatics, however, rapidly clear drug from these tissues, severely limiting access to the retina and vitreous humor [14,15].
Figure 1. . Fate of drug molecule after topical ocular administration.
Barriers to topical ophthalmic drug delivery
Topically administered therapeutic agents, targeting the inner ocular tissues, encounter static, dynamic and metabolic barriers that limit ocular bioavailability. These barriers can be classified as precorneal, corneal and the blood–ocular barriers [16,17].
Diffusional barriers
Precorneal barriers
The precorneal barrier affects both corneal and noncorneal pathways. Tear drainage forms a major precorneal barrier component [18–20]. The conjunctival cul-de-sac can accommodate approximately 7–10 μl fluid; thus, a significant portion of the topically administered eye drop, 35–50 μl, is lost [21]. Precorneal drainage not only causes the removal of the instilled formulation (drop/solution) but also reduces the corneal contact time for the formulation [22,23]. Patton reported an inverse correlation between instillation volume and bioavailability [24–26]. Also, the increased tear flow can lead to faster precorneal elimination and decreased efficacy. Osmolarity and pH of the formulation are two important parameters that can trigger tear fluid generation [27]. Additionally, tear fluid consist of proteins, to which the drug can bind, reducing the free drug concentration in the tear fluid [27]. Moreover, the multilayered tear film, with its hydrophilic and hydrophobic components [28], also presents a diffusion challenge by itself.
Diffusional barriers in the corneal pathway
Corneal barrier: Cornea is a multilayered barrier limiting drug penetration into the aqueous humor through the corneal pathway [15]. It has layers with alternative lipophilic and hydrophilic characteristics; the epithelium (contains 5–6 layers of epithelial cells) and endothelium are lipophilic in nature, whereas, stroma is hydrophilic in nature. Hence, the pharmaceutical active should have optimum hydrophilicity/lipophilicity, and other physicochemical characteristics such as MW, polar surface area, hydrogen bond donors and acceptors, for it to permeate efficiently across the cornea. Additionally, the corneal epithelial cells express tight junctions that restrict transcorneal paracellular diffusion, underlining the importance of the physicochemical characteristics and concentration gradients that govern transcellular, transcorneal diffusion. Efflux transporters, such as P-glycoprotein and multidrug-resistant proteins, expressed on the corneal epithelium [29] present additional barriers to transcorneal diffusion of their substrates [17,30–33]. Unlike the epithelial cells, corneal endothelium is leaky in nature, presenting little restriction to the movement of macromolecules between the stroma and aqueous humor [34]. Thus, the corneal epithelium acts as the major barrier to transcorneal diffusion.
Diffusional barriers in the conjunctival–scleral (noncorneal) pathway
Drug diffusion into the ocular tissue through the conjunctival–scleral pathway is also challenged. Although slightly leakier than the cornea [35], the conjunctival epithelium expresses efflux pumps that limit transconjunctival diffusion of its substrates. Since the conjunctiva is highly vascularized, unlike the cornea which is avascular, a significant fraction of the drug molecules penetrating across the conjunctival epithelium is lost into the systemic circulation. Moreover, tear dilutes the topically administered drop and the blinking action spreads the diluted formulation over the ocular surface, further decreasing the transconjunctival concentration gradient. Thus, formulations that settle in the conjunctival sac, and is not easily dispersed across the ocular surface, may utilize the conjunctival–scleral pathway more efficiently. Some recent publications using topical films/inserts seem to utilize this route of entry into the deeper ocular tissues from the surface [36].
Drug penetrating across the conjunctiva will reach the sclera from where it can reach the retina. The sclera is a poorly vascularized tissue comprising of collagen and mucopolysaccharides [37]. Scleral permeability is significantly higher than that of the cornea; however, considering that very little of the topically administered agent reaches the episcleral region, the concentration gradient across the sclera will be low. Some portion of the drug can also migrate laterally across the sclera, which will further decrease the trans-scleral concentration gradient. As a result, following topical instillation trans-scleral flux can be low, even though trans-scleral permeability may be greater than transcorneal permeability. Other factors affecting the scleral permeability include molecular radius, physicochemical properties and the surface charge of the active moiety [38–42].
The above discussion on the various barriers restricting ocular bioavailability of therapeutic agents following topical application is summarized in Table 1.
Table 1. . Physiological challenges to ocular drug delivery.
| Challenges | Effect | Ref. |
|---|---|---|
| Anatomy and physiological limitations | • Reduced precorneal residence time • Frequent dosing required; leading to a reduced patient compliance • Nasolacrimal drainage leads to drug loss • Systemic toxicity • Ocular toxicity |
[43–64] |
| Physicochemical limitations | • Reduce transcorneal flux • Poor drug solubility and permeability • Drug storage instability leading to reduced efficacy and increased cost |
[52,54–55,65–67] |
| Ocular and systemic toxicities | • Damage to the ocular tissues (retinal necrosis, loss of retinal ganglion cells, vitreous inflammation, corneal edema, neovascularization and inflammation) and occurrence of systemic toxicities (hepatotoxicity and nephrotoxicity) and side effects (gastrointestinal disturbances) observed. | [43,49–52,59,61–64,68–78] |
Metabolism in the ocular tissues
Enzymes (glutathione and related enzymes) form the defense system against foreign chemicals and oxidative stress, to protect the eyes. Glutathione and glucose-6-phosphate dehydrogenase is found in lens, cornea and retina [79]. Metabolizing enzymes, such as oxidoreductases and hydrolases, present in the eye can metabolize the administered drug and thus affect the response intensity and duration [80]. Various enzymes capable of metabolizing therapeutics agents present in the anterior segment ocular tissues are listed in the Table 2.
Table 2. . Various metabolizing enzymes expressed in anterior segment ocular tissues.
| Ocular tissue | Drug-metabolizing enzymes | Ref. |
|---|---|---|
| Cornea | CYP-1A/2B/2C/3A/4B1, NADPH reductases, ketone reductases, esterases, arylamine acetyltransferase, glutathione S-transferase. Aldehyde oxidase | [31,79,81–82] |
| Iris–ciliary bodies | Aldehyde oxidase, glutathione S-transferase, esterase, ketone reductase, CYP-1A/1B1/2B/2C/3A/4B1/39A1 | [79,81–82] |
| Lens | NaDC3, CYP2B, CYP2C, ketone reductase | [81–83] |
| Conjunctiva | Ketone reductase, aldehyde reductase, CYP-2b/3A/4B1, BCRP1 | [81,82] |
Elimination pathways
The faction of the topically administered agent that reaches the aqueous humor faces additional elimination mechanisms, in the form of aqueous humor outflow, which limits exposure to the iris–ciliary bodies and lens. Aqueous humor turnover leads to loss of the drug through the Canal of Schlemm and trabecular meshwork, and into the systemic circulation through the lymphatic circulation [84,85]. Diffusion into the microvasculature, present in the various parts of the eye, is another pathway via which the actives enter the systemic circulation [84,86]. Small lipophilic molecules are rapidly eliminated in comparison to large hydrophilic molecules ascribed to their ability to cross blood–aqueous barrier and enter the systemic circulation [87].
Nano-scaled drug-delivery systems for anterior segment diseases
The above sections highlight the challenges faced by formulation scientists in the development of effective, topical ophthalmic formulations. The low ocular bioavailability is thus not surprising considering the multiple physiological protective mechanisms encountered and underlines the need for specialized ophthalmic dosage forms.
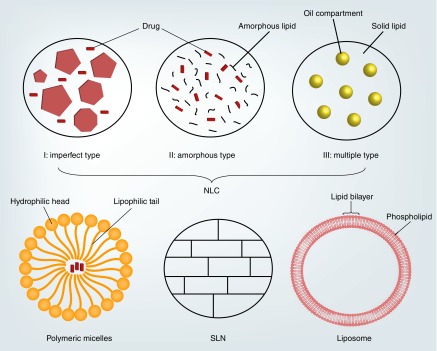
As is evident from the discussion above, the two major physiological hurdles encountered with topical application are retention at the site of administration and permeability coefficient of the therapeutic agent across the ocular tissues. Recently, nano-scaled drug-delivery systems have gained prominence as a noninvasive, topical, ocular drug-delivery platform, due to the various attributes of nanoparticles, such as improved precorneal retention through mucoadhesive characteristics, and active uptake by the corneal and conjunctival epithelia leading to enhanced ocular permeation and bioavailability. In this article, we review the literature with respect to the use of this formulation strategy with regards to drug delivery to the anterior segments of the eye. The polymer and lipid-based nanosystems are discussed separately in different sections. Figure 2 is an illustration depicting some common structures of micelles, liposomes and solid and nanostructured lipid nanoparticles. Although numerous studies exist in the literature, this review focuses on those that have in vivo data and have used control formulations to demonstrate improved delivery or efficacy.
Figure 2. . Illustration highlighting difference between polymeric micelles, liposome, solid lipid nanoparticle and nanostructured lipid carriers.
NLC: Nanostructured lipid carriers; SLN: Solid lipid nanoparticle.
Polymeric micelles & nanoparticles
Micelles are aggregates (supramolecular assembly) of polymers in aqueous solutions, with hydrophobic head group forming the core and hydrophilic chain facing the aqueous solution [88], therefore, making it feasible to modify the micelle surface by chemically conjugating various linkers to the hydrophilic chain. Surface coating determines the pharmacokinetic behavior of the polymeric systems [89].
Polymeric micelles have been studied to deliver genes (small RNA segments, viral vectors, plasmid DNA, etc.) and small molecules to the anterior segment tissues (various layers of cornea, aqueous humor) of the eye [90–93]. Gene therapy has shown promise in diseases affecting the anterior ocular segment, for example, dry eye syndrome, corneal clouding, corneal scarring, corneal neovascularization, angiogenesis and inflammation [92–94]. There have been a few reports on the delivery of oligonucleotides, siRNA and plasmid DNA to the corneal cells in vitro, with the help of nanocarriers [91–94].
The following sections look at some of the various polymers that have been used to design polymer-based micellar and nanoparticulate ophthalmic formulations. The role of the type, ratio and nature of the polymer/copolymers on the characteristics of the polymeric micelle and nanoparticles, and their interaction with the ocular membranes, therefore affecting the transmembrane delivery of the drug, is also discussed.
Ethylene glycol & D, L-lactide-based polymeric micelles
Tu et al. studied various weight ratios of the copolymers methoxy PEG (mPEG) to poly(D, L-lactide) and their effect on in vivo, in albino rabbits, and in vitro permeability across rabbit corneas [95]. They observed that a weight ratio of 80/20 of the mPEG to poly(D, L-lactide) significantly increased permeation of pirenzepine across the cornea (concentrations in aqueous humor) in vivo, which was consistent with the in vitro transcorneal permeability results.
In another set of studies, mPEG-hexyl-substituted poly(lactide) (mPEG-hPLA)-based micelles were used for the delivery of cyclosporine A to the cornea [96–98]. Based on the MTT – name based on the dye (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) – and clonogenic tests, in vitro and in vivo, mPEG-hPLA was considered to be biocompatible with the ocular tissues. The formulation outperformed the marketed formulation (Restasis) in pharmacodynamic studies (corneal transparency, edema and neovascularization), whereas results from the in vivo ocular biodistribution studies in rats were inconclusive. The cornea was the only tissue which had significantly higher cyclosporine delivered using the micellar system. Cyclosporine was not detected in other tissues of the eye, from both liposomal and marketed formulation [96–98].
Ethylene oxide & propylene oxide-based polymeric micelles
Pepic et al. demonstrated ocular delivery of pilocarpine (base and salt) using triblock copolymer Pluronic® F127 (poly(oxyethylene)/poly(oxypropylene)/poly(oxyethylene). The formulation contained 2% pilocarpine hydrochloride salt or 1.7% w/w pilocarpine base in a 2% Pluronic® F127 solution. Micelle size in water and buffer systems ranged from 15 to 30 nm. This was consistent with the fact that nonionic micellar systems are marginally affected in the presence of electrolytes. The micellar pilocarpine base formulation exhibited longer mitotic response time (tmr = 225 min) and area under the pharmacological activity curve (∼twofold) as compared with the micellar formulation of the pilocarpine salt (tmr = 180 min, AUC = 297 mm.min) [99]. This study underlines the role of the physicochemical characteristics of the molecule, in this case, the form of the drug (salt type, base) used in the nanoformulations, on the pharmacokinetic and pharmacodynamic response.
Liaw et al. demonstrated the use of poly(oxyethylene)/poly(oxypropylene)/poly(oxyethylene) (Pluronic® P105) nonionic copolymeric micelles of hydrodynamic diameter <160 nm for efficient delivery of stable plasmid DNA with lacZ gene, in vivo. The ζ-potential of the micelles was -4.4 ± 2 mV. In vivo gene transfer to the different layers of the cornea from the micellar ocular drops was achieved in mice and male albino New Zealand rabbits, which highlighted the potential of the block copolymer for DNA delivery. The DNA delivery efficiency was enhanced using the penetration enhancers, EDTA and cytochalasin B [91].
Gene delivery using topical eye drops was also reported by Tong et al. Two cornea-specific promoters: ketatin 12 and keratocan were formulated using Pluronic® P105 polymeric micelles. These proteins play a crucial role in developing and maintaining corneal transparency. β-Gal activity was the biomarker for transgene expression in the ocular tissues. The hydrodynamic diameter for the micelles ranged from 150 to 200 nm with unimodal distribution. The formulations were tested on both mice and rabbit animal models. From the in vivo tests, the authors concluded that the corneal epithelium and stroma-specific gene expression can be achieved with the help of cornea-specific promoters (ketatin 12 and keratocan) [92].
Taha et al. reported enhanced bioavailability of ciprofloxacin using polymeric micelles of Pluronic® F127. They observed a statistically significant increase in the area under the aqueous humor concentration versus time curve in comparison to the marketed formulation [100].
As discussed earlier, the contact time of the formulation with the surface ocular tissues plays a critical part in ocular drug bioavailability. Residence time can be prolonged by various means, one of them being the use of cationic polymer/s possessing mucoadhesive properties. One such naturally occurring cationic polymer which can improve ocular bioavailability is chitosan [101,102]. Chitosan is reported to have mucoadhesive property and acts as a permeation enhancer by reversibly opening epithelial tight junctions. Pepic et al. formulated a chitosan–Pluronic® F127 mixed micellar system with particle size ranging from 25 to 29 nm and ζ-potential 9 to 18 mV. Dexamethasone (0.48–0.56%) was loaded in the mixed micelle formed by Pluronic® F127 and chitosan. The authors considered the in vitro permeability enhancement of dexamethasone across Caco-2 cells and prolonged intraocular pressure increase in vivo as an indication of improved ocular bioavailability. In vivo pharmacokinetic studies in male albino New Zealand rabbits produced 2.4- and 1.4-folds increase in ocular bioavailability with Pluronic® F127 with 0.015 (w/v)% chitosan micelles, compared with dexamethasone ocular suspension and Pluronic® F127 micelles (no chitosan), respectively [102].
N-isopropylacrylamide-based polymers
Gupta et al. synthesized polymeric micelles (<100 nm particle diameter) composed of the co-polymers of N-isopropylacrylamide (NIPAAM), vinyl pyrrolidone and acrylic acid crosslinked with N, N’-methylene-bis-acrylamide to deliver ketorolac to the eye. The authors reported a twofold increase in transcorneal permeability of ketorolac over an aqueous ketorolac suspension at the same concentration [90]. The two ketorolac formulations were also studied in the albino rabbits, in vivo. The micellar formulation was observed to yield better therapeutic response, compared with the suspension, in terms of eyelid closure and polymorphonuclear leukocytes migration in the tear fluid.
Aspartamide & ethylene glycol-based polymers
Civiale et al. studied three copolymers of polyhydroxyethyl-aspartamide (PHEA), with side chains containing PEG and/or hexadecylamine (C16) (PHEA-PEG, PHEA-PEG-C(16) and PHEA-C(16), respectively) for its safety and efficacy for ocular drug delivery. They carried out the biophysical characterization of the PHEA-PEG, PHEA-C16 and PHEA-PEG-C16, the three PHEA derivatives. The physical characterization of the derivatized polymer was performed using Langmuir trough and micellar affinity capillary electrophoresis. From the micellar affinity capillary electrophoresis and Langmuir trough studies, they concluded that the polymers PHEA-C16 and PHEA-PEG-C16 were suitable for ocular drug delivery. The group conducted in vitro permeability studies across rabbit conjunctival and corneal epithelial cells. Netilmicin sulfate, dexamethasone alcohol and dexamethasone phosphate, the three molecules tested in this study, displayed higher permeation relative to their solution and suspension formulations. Confirmatory in vivo bioavailability studies were undertaken, in male albino rabbits, to evaluate the validity of the in vitro permeability data, comparing dexamethasone micelle to suspension. In vivo area under the concentration versus time curve for dexamethasone in aqueous humor was 40% higher from the micelles than from the dexamethasone suspension [103].
Usui et al. formulated polyion complex micelles of the porphyrin entrapped in dendrimer, for corneal neovascularization. The drug accumulation into the neovascularized cornea was greater in comparison to non-neovascularized cornea [104]. Entrapment of the drug in the dendrimer core reduced its activity, thereby reducing the toxicity associated with photodynamic therapy. The treatment was deemed to be safe and effective, ascribed to enhanced permeability and retention effect of the polyion complex micelle.
Ethylene glycol- & caprolactone-based polymers
Yingfang et al. synthesized mPEG-poly(ϵ-caprolactone) (mPEG-PCL) and formulated mPEG-PCL micelles dispersed in thermosensitive hydrogel for delivery of pimecrolimus to treat Keratoconjunctivitis sicca, also known as dry eye syndrome. PEG-400 is widely used in ophthalmic formulations to treat dry, irritated eyes, although, effect of PEG chain length on irritation and toxicity is unknown [105]. There was a significant rise in the tear fluid generation on the 20th day, in vivo in mice, in comparison to artificial tear formulation, and a thermosensitive hydrogel formulation. Histological examination showed no signs of corneal epithelium damage, in vivo in rabbits [106]. Other reports suggest that the polymer mPEG-PCL can be chemically linked to numerous compounds (e.g., folate, cholesterol, etc.) for anticancer targeting [107]. The micelles exhibited low cellular cytotoxicity in human corneal epithelial cells, human lens epithelial cells and retinal pigment epithelium [106].
Guo et al. showed high corneal uptake of cyclosporine-loaded polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymeric micelles in rabbits [108]. The formulation was deemed to be safe from the in vitro cytotoxic assay and was well tolerated in the rabbits. The particle size of the nanoparticles ranged from 70 to 78 nm.
Cyanoacrylate-based polymers
Losa et al. studied enhancement of the corneal permeation of a hydrophilic drug (amikacin sulfate) entrapped in polycyanoacrylate nanoparticles. They studied three nanoparticle formulations varying in the type of the stabilizing agent – dextran 70,000, synperonic® F68 and sodium lauryl sulfate. The particle size of the formulations consisting of either dextran 70,000 or sodium lauryl sulfate as the stabilizer ranged from 210 to 235 nm, while, significantly lower particle size (80 ± 1.3 nm) was observed for formulation with synperonic F68 (a polyol detergent) as the stabilizing agent. The ζ-potential ranged from -4 to -6 mV. Amikacin concentration achieved in aqueous humor and cornea for the nanoparticles with dextran was significantly higher in comparison to nanoparticles with other stabilizing agents and control amikacin sulfate solution, in vivo in rabbits [109].
D,L-lactide-co-glycolide & ethylene glycol-based polymers
Musumeci et al. investigated melatonin-loaded poly(D,L-lactide-co-glycolide) (PLGA) and PLGA-PEG nanoparticles with particle size ranging from 100 to 400 nm for sustained release of melatonin. Melatonin-loaded PLGA-PEG nanoparticles demonstrated extended precorneal residence of melatonin as evident from the lowering of the intraocular pressure in rabbit eye for up to 8 h, with a maximum intraocular pressure (IOP) reduction of 5 mmHg. Aqueous solution demonstrated IOP-lowering effect for up to 4 h only. The duration of IOP reduction was, thus, significantly greater with the melatonin PLGA nanoparticles than that observed with the solution formulation [110]. In another study, Warsi et al. prepared dorzolamide-loaded PLGA nanoparticles utilizing D-α-tocopheryl PEG 1000 succinate (TPGS) and polyvinyl alcohol as the emulsifiers. TPGS demonstrated 77-fold greater emulsifying efficiency. The nanoparticles had significantly higher transcorneal permeation in vitro, approximately twofold for nanoparticles with TPGS and approximately 2.5-fold for nanoparticles with polyvinyl alcohol, in comparison to dorzolamide solution. The three dorzolamide formulations – solution, nanoparticle with TPGS and nanoparticle with polyvinyl alcohol, showed IOP reduction of 15.8, 22.75 and 16%, respectively. The authors hypothesize that the increase in permeation and IOP reduction could be attributed to the capability of TPGS to inhibit P-glycoprotein [111].
Thus, a variety of polymers, alone and in combination, have been investigated with respect to ocular drug delivery in vivo. Table 3 consolidates the above discussion.
Table 3. . Summary of various polymer-based nano scale drug-delivery systems studied in vivo.
| Polymers | Drug/dye | Summary | Ref. |
|---|---|---|---|
| Methoxy PEG and poly(D,L-lactide)-based micelles | Pirenzepine | mPEG-hPLA 80/20 weight ratio showed significantly higher permeability, both in vivo and in vitro, in comparison to 50/50 and 40/60 weight ratios | [95] |
| Methoxy PEG-hexyl-substituted poly(lactide) | Cyclosporine | The formulation was nontoxic (in vitro and in vivo). It had better pharmacokinetic and pharmacodynamic profile in comparison to marketed preparation | [112–114] |
| Pluronic® F127 | Pilocarpine | The mitotic response time was significantly increased in comparison to pilocarpine salt solution | [99] |
| N-isopropylacrylamide, vinyl pyrrolidone and acrylic acid cross-linked with N, N’-methylene bis-acrylamide-based micelles | Ketorolac | A twofold increase in the transcorneal permeation was observed, in comparison to suspension was observed. The micelles had better therapeutic response in comparison to suspension, in vivo in rabbits | [90] |
| Micelle based on polyhydroxyethyl-aspartamide with PEG and/or hexadecylamine side chain | Dexamethasone and netilmicin | Transcorneal permeation was significantly increased in the micelles in comparison to suspension | [103] |
| PEO-PPO-PEO-based micelles | LacZ gene | The gene was delivered to the cornea, in vivo in rabbits | [91] |
| PEO-PPO-PEO-based micelles | Ketatin 12 and Keratocan | The gene was delivered to the cornea in the mice and rabbits | [92] |
| Pluronic® F127 and chitosan micelles | Dexamethasone | The pluronic® F127 – chitosan-based micelles demonstrated a 2.4-and 1.4-fold increase in bioavailability in comparison to ocular suspension and Pluronic® F127-based micelles, respectively | [102] |
| Polyion complex micelles | Dendrimer porphyrin | Increased drug accumulation in neovascularized tissues was observed in comparison to non-neovascularized tissue | [104] |
| Pluronic® F127 | Ciprofloxacin | The drug-loaded Pluronic® F127 micelles had statistically higher area under the concentration in aqueous humor vs time curve in comparison to marketed formulation | [100] |
| Polycyanoacrylate polymeric nanoparticles | Amikacin sulfate | Greater ocular disposition was achieved with polymeric nanoparticles having dextran as stabilizer in comparison to other stabilizer and the amikacin control solution | [109] |
| Chitosan hydrochloride and N-carboxymethyl chitosan-based nanoparticles | Ofloxacin | Chitosan hydrochloride showed greater transcorneal penetration as well as intraocular penetration in comparison to carboxymethyl chitosan and marketed eye drops | [115] |
| PLGA and PLGA-PEG nanoparticles | Melatonin | Melatonin-loaded PLGA-PEG nanoparticles demonstrated extended precorneal residence of melatonin evident by lowering intraocular pressure in rabbit eye up to 8 h with maximum IOP reduction of 5 mmHg, which was significantly lower than melatonin PLGA nanoparticles and solution | [110] |
| Methoxy PEG Poly(caprolactone) | Pimecrolimus | Methoxy PEG-poly(ϵ-caprolactone) micelles were found to be superior in the in vitro and in vivo experiments | [106] |
| Positively charged polymer (PEG)-poly(ε-caprolactone)-g-polyethyleneimine-based micelles | Fluorescein diacetate | The micelles demonstrated better corneal penetration as compared with the control nanoparticles and microparticles, which was evident from in vivo studies | [116] |
| PLGA nanoparticles | Dorzolamide | Dorzolamide nanoparticle showed promising IOP-lowering effect as compared with dorzolamide solution. TPGS being a P-glycoprotein inhibitor might have significantly enhanced permeation across the goat eye corneas and reduced intraocular pressure through cornea in comparison to dorzolamide solution, in vivo in albino rabbit | [111] |
| Polyvinyl caprolactam-polyvinyl acetate-PEG graft copolymeric micelles | Cyclosporine | The particle size of the nanoparticles ranged from 70 to 78 nm. The formulation was also found to be safe from the in vitro cytotoxic assay, and had excellent tolerance in rabbits | [108] |
PEO-PPO-PEO: Poly(oxyethylene)/poly(oxypropylene)/poly(oxyethylene); PLGA: Poly(D,L-lactide-co-glycolide); TPGS: D-α-tocopheryl PEG 1000 succinate.
Lipid nanoparticles
Lipids can form various nanostructures, such as micelles, reverse micelles, liposomes, solid lipid particles, nanostructured lipid carriers, based on the lipid combinations used and the other formulation components selected [117]. Lipids are abundantly present in the body and various biodegradation pathways exist for lipids. Safety and efficacy of the ointments and emulsions, which are lipid-based delivery systems, have been established and marketed since many years, therefore, lipid nanoparticles are expected to be biocompatible and safe [118]. The main advantages of the lipid nanoparticles over polymeric systems are low toxicity, solvent-free production methods and cost. Recently, modified lipids have been widely used to form lipid nanoparticles.
Liposomes
Liposomes are biodegradable, biocompatible vesicular nano systems, typically composed of phosphatidylcholine, cholesterol and lipid-conjugated hydrophilic polymers, and have the flexibility to load both hydrophilic and lipophilic drug molecules. Surface-modified liposomes, for example, for mucoadhesion or enhanced penetration, have also been studied.
Charge has been shown to be a critical parameter. A study showed corneal permeation, in decreasing order, of small cationic unilamellar vesicles (SUV+), multilamellar anionic vesicles (MLV-), small anionic unilamellar vesicles (SUV-), SUV, multilamellar anionic vesicles and drug suspension, across excised rabbit cornea [119].
Singh and Mezei formulated liposomes containing triamcinolone acetonide. They observed a twofold enhancement in drug concentrations in the cornea as well as in the aqueous humor, in rabbits, in comparison to the suspension formulation. The liposome maintained the higher drug concentration in the aqueous humor for 5 h [120,121]. Reports from the same group using dihydrostreptomycin sulfate, a hydrophilic drug, loaded in the liposomal formulation, however, showed no improvement in transcorneal permeation in comparison to the control drug solution. To explain this observation, the authors proposed that the mechanism by which the liposomes interact with the corneal cell membrane is by surface adsorption and surface lipid exchange. The observation that liposomal formulations enhanced permeation of the lipophilic molecule, triamcinolone acetonide, but not that of the hydrophilic compound, dihydrostreptomycin sulfate, may be because the hydrophilic compound is not exchanged with the corneal epithelial lipid membrane but rather released into the precorneal tear fluids [122].
In another study, Shihui et al. formulated timolol-loaded liposomes incorporated into in situ gels using gellan gum. Transcorneal timolol permeation was 1.9-times greater than that from the marketed eye drops. The IOP-lowering effect with the liposomal timolol formulation was observed for 240 min, which was significantly greater than that with the marketed preparation, demonstrating the potential of the liposome/in situ gel combination formulations in ocular drug delivery [123].
A summary of the various reports on topical liposomal formulations for enhancing ocular permeation is presented in Table 4.
Table 4. . Summary of various lipid nano formulations evaluated in vivo for drug delivery to the anterior segment of the eye.
| Formulation/lipids | Drug | Summary | Ref. |
|---|---|---|---|
| Liposomes | |||
| DL-dipalmitoyl-phosphatidyl choline and cholesterol | Triamcinolone acetonide | A twofold higher drug concentration was observed in cornea as well as aqueous humor in comparison to suspension | [121] |
| DL-dipalmitoyl-phosphatidyl choline and cholesterol | Dihydro-Streptomycin sulfate | Liposome showed no significant improvement in permeability, in vivo, comparison to the control | [122] |
| L-α-phosphatidylcholine, cholesterol, stearylamine and dicetyl phosphate | Ciprofloxacin hydrochloride | Chitosan-coated liposomal formulation had longer in vivo retention time in male albino New Zealand rabbits in comparison to the marketed Ciprocin® drops | [124] |
| Soy-phosphatidyl choline and cholesterol | Timolol maleate | Timolol maleate liposome-loaded gellan gum gels had ∼twofold greater transcorneal permeation in comparison to the marketed formulation | [123] |
| Solid lipid nanoparticles/nanostructured lipid carriers | |||
| Compritol® ATO 555 and palmitic acid | Voriconazole | The AUC for concentration in aqueous humor over the 12-h time period, was ∼twofold greater for SLN in comparison to drug suspension | [125] |
| Compritol ATO-888, Gelucire®50/13, and Precirol®ATO-5 | Gatifloxacin | Gatifloxacin concentration in aqueous humor was found to be ∼threefold in comparison to marketed preparation | [126] |
| Soya-phospholipid SL-100 and triglyceride | Baicalin | Transcorneal permeation was (rabbit eyes) ∼1.5-times higher for SLN in comparison to solution, whereas, AUC was ∼four-times and Cmax ∼5.5-times for SLN in comparison to solution. Formulation was nonirritating to the eye | [127] |
| Glyceryl monostearate and lecithin | Methazolamide | The maximum intraocular pressure reduction was 42%, which was significantly higher in comparison to marketed brinzolamide marketed preparation (38%), in vivo in rabbits | [128] |
| Compritol ATO 888, Stearylamine and gelucire 44/14 | Ibuprofen | The transcorneal permeation across the rabbit cornea for ibuprofen-loaded NLC was three–four-times in comparison to eye drops. From the in vivo aqueous humor kinetic study, AUC and Cmax for the NLC were twice in comparison to eye drops | [129] |
NLC: Nanostructured lipid carrier; SLN: Solid lipid nanoparticle.
Solid lipid nanoparticles
Solid lipid nanoparticles (SLNs) have been widely used for drug delivery via oral, topical, ophthalmic, parenteral and other routes [130–142]. SLNs suffer from some disadvantages such as burst release with hydrophilic drugs and low drug loading owing to the solid crystalline state of the nanoparticles. These shortcomings led to the development of second-generation lipid nanoparticles, the nanostructured lipid carriers (NLC). NLCs differ from SLNs in that liquid lipid is mixed with solid lipid which prevents crystallization of lipids (Figure 2). This incorporation leads to the formation of one of the following: imperfect NLC, multiple type NLC or structureless NLCs. The type of NLC formed depends on the concentration of liquid lipid utilized and method of preparation employed. Inclusion of the liquid lipid in the NLCs enhances drug solubility, thereby increasing drug loading significantly, and reduces the crystallinity of the solid lipid, consequently reducing the problem of drug expulsion. Recently, a few studies have demonstrated the ability of the SLNs to permeate across the cornea into the aqueous humor (Table 4).
Kumar and Sinha formulated voriconazole-loaded SLNs and studied ocular permeation of voriconazole, ex vivo (excised rabbit corneas) and in vivo in rabbits. They formulated SLN using Compritol® ATO 555 and palmitic acid. The particle size of the formulation ranged from 234 to 343 nm with entrapment efficiency ranging from 61.91 ± 2.04% to 84.25 ± 1.11%. The AUC for concentration in aqueous humor over the 12-h time period, was approximately twofold greater for SLN in comparison to drug suspension [125].
Similarly, in another study, permeation of gatifloxacin was increased using SLN formulation, in vivo in rabbits [126]. The gatifloxacin area under the aqueous humor concentration – time curve was approximately threefold higher in comparison to Gate®-marketed eye drops. The authors ascribed this to the changes in the elimination from the aqueous humor (∼half) and a significant increase in the Cmax.
In another study by Cavalli et al., tobramycin salt–ion pair complex-loaded SLNs were compared with solution formulations. Aqueous humor pharmacokinetic parameters were significantly better for the SLNs in comparison to tobramycin solution, in vivo in rabbits [143]. Tobramycin AUC was found to be significantly higher (∼four-times) and Cmax was found to be about twofold higher for the SLNs in comparison to the solution. From the precorneal retention study, using a fluorescent probe tagged to the SLN, the residence time in the cul-de-sac for SLN (90 min) was higher in comparison to solution (30 min).
Similarly, baicalin, methazolamide and ibuprofen-loaded SLNs showed significantly better transcorneal permeation and/or pharmacokinetic properties (AUC, Cmax) in comparison to the conventional dosage forms (suspensions, solutions) which were used as comparators [127–128,135,144–146].
In a recent study by Balguri et al., the advantages of SLNs and NLCs in terms of drug loading and ocular penetration were demonstrated. Indomethacin was used as the therapeutic candidate whose ocular distribution was evaluated following topical application. The studies revealed that the NLC formulations were capable of higher drug loading and achieved significantly higher concentrations in all the ocular tissues tested [134]. The data also demonstrated that surface modification of the NLCs with PEG improved ocular penetration.
Other studies have also demonstrated that surface modification of the NLCs can improve in vivo biodistribution of the drugs [134,147–149]. Shen et al. formulated thiolated nanostructured lipid carriers for ocular delivery of cyclosporine A [147]. They compared NLC, PEGylated NLC, thiolated NLC and oily solution of cyclosporine A in the pharmacokinetic study, by analyzing their tear fluid, cornea, iris ciliary and aqueous humor at various time points, in vivo in rabbits. Cys-NLC demonstrated superior pharmacokinetic behavior (all tissues tested) followed by PEGylated NLC in comparison to all other formulations.
Cubosomes
Cubosomes are liquid crystalline nanoparticles of the amphiphilic lipids with definite carbon chain length. The cubosome have higher physicochemical stability in comparison to liposome due to strong electric repulsion. The microstructure of the cubosomes is similar to the biological membrane, which leads to enhanced permeation. The ability of cubosome to load is ascribed to lipid bilayer with its high surface area [150,151]. Li et al. formulated cubosome of glycerol monoolein and poloxamer 407 loaded with pilocarpine nitrate for glaucoma therapy [152]. They observed approximately two-times higher transcorneal flux across the rabbit corneas in comparison to the marketed eye drops. To further compare efficacy of the cubosome in comparison to the marketed eye drops they performed aqueous humor kinetics in anesthetized rabbits and pharmacodynamic studies measuring IOP change over time. The cubosome had prolonged the retention time (∼two-times) in comparison to marketed eye drops. The cubosomes had significantly higher effect on IOP reduction (∼50% IOP reduction) in comparison to the marketed eye drops (∼41%). Similarly, Huang et al. formulated cubosomes of glycerol monoolein and poloxamer 407 loaded with timolol maleate [150]. They observed approximately 3.5-times higher cumulative drug permeation across the rabbit corneas in comparison to the marketed eye drops. The cubosome had prolonged the retention time (∼two-times) in comparison to marketed eye drops. The cubosomes had significantly higher effect on IOP reduction (∼36% IOP reduction) in comparison to the marketed eye drops (∼15%). Cubosomes were deemed to be safe in the above reports by the authors on the basis of draize test, corneal hydration levels and corneal histology.
Conclusion & future perspective
Advances in ophthalmic dosage forms, in conjunction with a better understanding of the physiological challenges and with the discovery of new therapeutic modalities, have aided in the development of approaches that can mitigate the challenges associated with the pharmacotherapy of the anterior segment ocular diseases. Much of it can be attributed to the integration of nanotechnology into the development of topical ocular dosage forms intended for the treatment of diseases such as cataract, glaucoma, keratitis, etc.
Despite the widespread research in this area, new polymeric or lipid-based nano-scaled ophthalmic drug-delivery systems have not reached the market yet. This is probably because of many unanswered questions in this area. Future research needs to yield a better understanding of the mechanism of penetration of these nanoparticles and effect of physicochemical characteristics and formulation parameters such as particle size; metabolism of the polymer and lipid conjugates in the ocular tissues; ocular toxicity of the polymers and their breakdown products; release of the drug in the ocular tissues; tissue response to the lipid and polymer conjugates; chronic toxicity; sterilization; and formulation stability. Moreover, many of the nano dosage forms involve the use of organic solvents during the manufacturing process. Organic solvent concentrations need to meet the residual solvent limits or avoided, if possible [153,154]. Another critical area that needs scrutiny is the effect of the surfactants in these formulations on the ocular tissues [154]. Thus, a concerted approach is needed before these promising nanotechnologies can be successfully used in the treatment of ocular diseases.
Executive summary.
Ocular diseases, affecting the anterior and posterior ocular segments, are on a steady rise, and if left untreated, or inappropriately treated, could lead to vision loss.
Topical application is the most preferred route for ocular diseases due to the noninvasive and higher patient compliance attributes associated with it.
However, for the successful delivery of drugs via the topical route, there is a need for overcoming the pre- and postcorneal anatomical and physiological barriers.
To overcome these barriers, various novel nano-scaled formulation approaches, such as lipid nanoparticles and polymeric micelles, have been extensively pursued, with intent to target the anterior and posterior ocular segment diseases.
Among these, nano systems based on polymeric and lipid-based micelles, liposomes and nanoparticle therapeutics have shown promise in terms of improving treatment outcomes in anterior segment diseases.
Despite promising developments in nanotherapeutics for ocular delivery, their transition from the preclinical to the clinical setting has been slow; majorly owing to scale-up challenges associated with the manufacture of nanoparticles and the lack of a complete and coherent understanding of the ocular toxicities caused by them.
The choice of lipids/polymers/surfactants, breakdown products, stability, sterilization and analysis of the cellular response to the nano systems, on acute and chronic administration, needs to be analyzed in depth.
Concentrated research focused on understanding and overcoming the challenges associated with the aforementioned areas of nanotherapeutics would ensure complete and successful transition of the nanotherapeutic dosage forms in the pharmacotherapy of ocular diseases.
Footnotes
Financial & competing interests disclosure
This project was supported by grants 1R01EY022120–01A1 from the National Eye Institute and P20GM104932 from the National Institute of General Medical Sciences, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Nateyeinstitute. Statistics and data. 2017.
- 2.Perlman JI. Ocular disease: mechanisms and management. JAMA. 2011;306(1):101–101. [Google Scholar]
- 3.Patil A, Majumdar S. Echinocandins in ocular therapeutics. J. Ocul. Pharmacol. Ther. 2017;33(5):340–352. doi: 10.1089/jop.2016.0186. [DOI] [PubMed] [Google Scholar]
- 4.Patil A, Lakhani P, Majumdar S. Current perspectives on natamycin in ocular fungal infections. J. Drug Deliv. Sci. Technol. 2017;41:206–212. [Google Scholar]
- 5.Taskar P, Tatke A, Majumdar S. Advances in the use of prodrugs for drug delivery to the eye. Expert Opin. Drug Deliv. 2017;14(1):49–63. doi: 10.1080/17425247.2016.1208649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Causes of blindness and visual impairment. 2017. www.who.int/blindness/causes/en/
- 7.American Academy of Ophthalmology. Eye health statistics. 2017. www.aao.org/newsroom/eye-health-statistics
- 8.WHO. Priority eye diseases. 2014. www.who.int/blindness/causes/priority/en/index1.html
- 9.Patil A, Singh S, Opere C, Dash A. Sustained-release delivery system of a slow hydrogen sulfide donor, GYY 4137, for potential application in glaucoma. AAPS PharmSciTech. 2017;18(6):2291–2302. doi: 10.1208/s12249-017-0712-6. [DOI] [PubMed] [Google Scholar]
- 10.Patel A, Cholkar K, Agrahari V, Mitra AK. Ocular drug delivery systems: an overview. World J. Pharmacol. 2013;2(2):47–64. doi: 10.5497/wjp.v2.i2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim NJ, Harris A, Elghouche A, Gama W, Siesky B. Ocular permeation enhancers. In: Pathak Y, Sutariya V, Hirani AA, editors. Nano-Biomaterials For Ophthalmic Drug Delivery. Springer International Publishing Cham; Switzerland: 2016. pp. 177–209. [Google Scholar]
- 12.Kaur IP, Smitha R. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev. Ind. Pharm. 2002;28(4):353–369. doi: 10.1081/ddc-120002997. [DOI] [PubMed] [Google Scholar]
- 13.Le Bourlais C, Acar L, Zia H, Sado PA, Needham T, Leverge R. Ophthalmic drug delivery systems – recent advances. Prog. Retin. Eye Res. 1998;17(1):33–58. doi: 10.1016/s1350-9462(97)00002-5. [DOI] [PubMed] [Google Scholar]
- 14.Huang HS, Schoenwald RD, Lach JL. Corneal penetration behavior of β-blocking agents II: assessment of barrier contributions. J. Pharm. Sci. 1983;72(11):1272–1279. doi: 10.1002/jps.2600721109. [DOI] [PubMed] [Google Scholar]
- 15.Levin L, Nilsson S, Hoeve JV, Wu S, Kaufman P, Alm A. Adler's Physiology Of The Eye: Clinical Application (11th Edition) Elsevier; NY, USA: 2011. [Google Scholar]; • Talks extensively about the anatomy and physiology of the eye.
- 16.Mitra AK. Ophthalmic Drug Delivery Systems. Taylor & Francis; FL, USA: 2003. Fundamental considerations; pp. 1–60. [Google Scholar]; • Has extensive information on various aspects of ocular drug delivery.
- 17.Mitra AK. Ocular Transporters and Receptors: Their Role in Drug Delivery. Woodhead publishing; PA, USA: 2013. [Google Scholar]
- 18.Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348–360. doi: 10.1208/s12248-010-9183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghate D, Edelhauser HF. Ocular drug delivery. Expert Opin. Drug Deliv. 2006;3(2):275–287. doi: 10.1517/17425247.3.2.275. [DOI] [PubMed] [Google Scholar]
- 20.Schoenwald RD. Ocular drug delivery. Pharmacokinetic considerations. Clin. Pharmacokinet. 1990;18(4):255–269. doi: 10.2165/00003088-199018040-00001. [DOI] [PubMed] [Google Scholar]
- 21.John Lang RR, Rajni Jani. Opthalmic Preparations. In: Remington JP, editor. Remington: the Science And Practice Of Pharmacy. Lippincott Williams & Wilkins; MD, USA: 2006. pp. 873–900. [Google Scholar]
- 22.Dursun D, Monroy D, Knighton R, et al. The effects of experimental tear film removal on corneal surface regularity and barrier function. Ophthalmology. 2000;107(9):1754–1760. doi: 10.1016/s0161-6420(00)00273-6. [DOI] [PubMed] [Google Scholar]
- 23.Nejima R, Miyata K, Tanabe T, et al. Corneal barrier function, tear film stability, and corneal sensation after photorefractive keratectomy and laser in situ keratomileusis. Am. J. Ophthalmol. 2005;139(1):64–71. doi: 10.1016/j.ajo.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 24.Chrai SS, Makoid MC, Eriksen SP, Robinson JR. Drop size and initial dosing frequency problems of topically applied ophthalmic drugs. J. Pharm. Sci. 1974;63(3):333–338. doi: 10.1002/jps.2600630304. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed I. Ophthalmic Drug Delivery Systems (2nd Edition) CRC Press; NY, USA: 2003. The noncorneal route in ocular drug delivery; pp. 335–363. [Google Scholar]
- 26.Patton TF. Pharmacokinetic evidence for improved ophthalmic drug delivery by reduction of instilled volume. J. Pharm. Sci. 1977;66(7):1058–1059. doi: 10.1002/jps.2600660746. [DOI] [PubMed] [Google Scholar]
- 27.Janssen PT, Van Bijsterveld OP. Origin and biosynthesis of human tear fluid proteins. Invest. Ophthalmol. Vis. Sci. 1983;24(5):623–630. [PubMed] [Google Scholar]
- 28.Svitova TF, Lin MC. Tear lipids interfacial rheology: effect of lysozyme and lens care solutions. Optom. Vis. Sci. 2010;87(1):10–20. doi: 10.1097/OPX.0b013e3181c07908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dey S, Mitra AK. Transporters and receptors in ocular drug delivery: opportunities and challenges. Expert Opin. Drug Deliv. 2005;2(2):201–204. doi: 10.1517/17425247.2.2.201. [DOI] [PubMed] [Google Scholar]
- 30.Dey S, Patel J, Anand BS, et al. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest. Ophthalmol. Vis. Sci. 2003;44(7):2909–2918. doi: 10.1167/iovs.02-1142. [DOI] [PubMed] [Google Scholar]
- 31.Karla PK, Earla R, Boddu SH, Johnston TP, Pal D, Mitra A. Molecular expression and functional evidence of a drug efflux pump (BCRP) in human corneal epithelial cells. Curr. Eye Res. 2009;34(1):1–9. doi: 10.1080/02713680802518251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karla PK, Pal D, Quinn T, Mitra AK. Molecular evidence and functional expression of a novel drug efflux pump (ABCC2) in human corneal epithelium and rabbit cornea and its role in ocular drug efflux. Int. J. Pharm. 2007;336(1):12–21. doi: 10.1016/j.ijpharm.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Zhang JJ, Koppel H, Jacob TJ. P-glycoprotein regulates a volume-activated chloride current in bovine non-pigmented ciliary epithelial cells. J. Physiol. 1996;491(Pt 3):743–755. doi: 10.1113/jphysiol.1996.sp021254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuft SJ, Coster DJ. The corneal endothelium. Eye (Lond.) 1990;4(Pt 3, 3):389–424. doi: 10.1038/eye.1990.53. [DOI] [PubMed] [Google Scholar]
- 35.Saha P, Yang JJ, Lee VH. Existence of a p-glycoprotein drug efflux pump in cultured rabbit conjunctival epithelial cells. Invest. Ophthalmol. Vis. Sci. 1998;39(7):1221–1226. [PubMed] [Google Scholar]
- 36.Balguri SP, Adelli GR, Tatke A, Janga KY, Bhagav P, Majumdar S. Melt-cast noninvasive ocular inserts for posterior segment drug delivery. J. Pharm. Sci. 2017;106(12):3515–3523. doi: 10.1016/j.xphs.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamalainen KM, Kananen K, Auriola S, Kontturi K, Urtti A. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest. Ophthalmol. Vis. Sci. 1997;38(3):627–634. [PubMed] [Google Scholar]
- 38.Ambati J, Canakis CS, Miller JW, et al. Diffusion of high molecular weight compounds through sclera. Invest. Ophthalmol. Vis. Sci. 2000;41(5):1181–1185. [PubMed] [Google Scholar]
- 39.Cruysberg LPJ, Nuijts RMMA, Geroski DH, Koole LH, Hendrikse F, Edelhauser HF. In vitro human scleral permeability of fluorescein, dexamethasone-fluorescein, methotrexate-fluorescein and rhodamine 6G and the use of a coated coil as a new drug delivery system. J. Ocul. Pharmacol. Ther. 2002;18(6):559–569. doi: 10.1089/108076802321021108. [DOI] [PubMed] [Google Scholar]
- 40.Maurice DM, Polgar J. Diffusion across the sclera. Exp. Eye Res. 1977;25(6):577–582. doi: 10.1016/0014-4835(77)90136-1. [DOI] [PubMed] [Google Scholar]
- 41.Prausnitz MR, et al. Schools of Chemical E, Biomedical Engineering GIOTaG. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J. Pharm. Sci. 2017;87(12):1479–1488. doi: 10.1021/js9802594. [DOI] [PubMed] [Google Scholar]
- 42.Dunlevy JR, Rada JA. Interaction of lumican with aggrecan in the aging human sclera. Invest. Ophthalmol. Vis. Sci. 2004;45(11):3849–3856. doi: 10.1167/iovs.04-0496. [DOI] [PubMed] [Google Scholar]
- 43.Goldblum D, Rohrer K, Frueh B, Theurillat R, Thormann W, Zimmerli S. Ocular distribution of intravenously administered lipid formulations of amphotericin B in a rabbit model. Antimicrob. Agents Chemother. 2002;46(12):3719–3723. doi: 10.1128/AAC.46.12.3719-3723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen T, Sauvageon-Martre H, Brossard D, et al. Amphotericin B eye drops as a lipidic emulsion. Int. J. Pharm. 1996;137(2):249–254. [Google Scholar]
- 45.Barza M, Baum J, Tremblay C, Szoka F, D'amico D. Ocular toxicity of intravitreally injected liposomal amphotericin B in rhesus monkeys. Am. J. Ophthalmol. 1985;100(2):259–263. doi: 10.1016/0002-9394(85)90791-3. [DOI] [PubMed] [Google Scholar]
- 46.Tremblay C, Barza M, Szoka F, Lahav M, Baum J. Reduced toxicity of liposome-associated amphotericin B injected intravitreally in rabbits. Invest. Ophthalmol. Vis. Sci. 1985;26(5):711–718. [PubMed] [Google Scholar]
- 47.Das S, Suresh P. Nanosuspension: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to amphotericin B. Nanomedicine. 2011;7(2):242–247. doi: 10.1016/j.nano.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Das S, Suresh P, Desmukh R. Design of Eudragit RL 100 nanoparticles by nanoprecipitation method for ocular drug delivery. Nanomedicine. 2010;6(2):318–323. doi: 10.1016/j.nano.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Chhonker Y, Prasad Y, Chandasana H, et al. Amphotericin-B entrapped lecithin/chitosan nanoparticles for prolonged ocular application. Int. J. Biol. Macromol. 2015;72:1451–1458. doi: 10.1016/j.ijbiomac.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 50.Bhatta R, Chandasana H, Chhonker Y, et al. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: in vitro and pharmacokinetics studies. Int. J. Pharm. 2012;432(1):105–112. doi: 10.1016/j.ijpharm.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 51.Chandasana H, Prasad Y, Chhonker Y, et al. Corneal targeted nanoparticles for sustained natamycin delivery and their PK/PD indices: an approach to reduce dose and dosing frequency. Int. J. Pharm. 2014;477(1):317–325. doi: 10.1016/j.ijpharm.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 52.Paradkar M, Parmar M. Formulation development and evaluation of Natamycin niosomal in-situ gel for ophthalmic drug delivery. J. Drug Deliv. Sci. Technol. 2017;39:113–122. [Google Scholar]
- 53.Silva G, Almeida A, Fernandes-Cunha G, et al. Safety and in vivo release of fluconazole-loaded implants in rabbits’ eyes. J. Drug Deliv. Sci. Technol. 2016;35:323–326. [Google Scholar]
- 54.Fetih G. Fluconazole-loaded niosomal gels as a topical ocular drug delivery system for corneal fungal infections. J. Drug Deliv. Sci. Technol. 2016;35:8–15. [Google Scholar]
- 55.Abdelbary G, Amin M, Zakaria M. Ocular ketoconazole-loaded proniosomal gels: formulation, ex vivo corneal permeation and in vivo studies. Drug Deliv. 2017;24(1):309–319. doi: 10.1080/10717544.2016.1247928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmed T, Aljaeid B. A potential in situ gel formulation loaded with novel fabricated poly(lactide-co-glycolide) nanoparticles for enhancing and sustaining the ophthalmic delivery of ketoconazole. Int. J. Nanomedicine. 2017;12:1863–1875. doi: 10.2147/IJN.S131850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kakkar S, Karuppayil S, Raut J, et al. Lipid-polyethylene glycol based nano-ocular formulation of ketoconazole. Int. J. Pharm. 2015;495(1):276–289. doi: 10.1016/j.ijpharm.2015.08.088. [DOI] [PubMed] [Google Scholar]
- 58.Grossman R, Lee D. Transscleral and transcorneal iontophoresis of ketoconazole in the rabbit eye. Ophthalmology. 1989;96(5):724–729. doi: 10.1016/s0161-6420(89)32832-6. [DOI] [PubMed] [Google Scholar]
- 59.Jaiswal M, Kumar M, Pathak K. Zero order delivery of itraconazole via polymeric micelles incorporated in situ ocular gel for the management of fungal keratitis. Colloids Surf. B Biointerfaces. 2015;130:23–30. doi: 10.1016/j.colsurfb.2015.03.059. [DOI] [PubMed] [Google Scholar]
- 60.Ahuja M, Verma P, Bhatia M. Preparation and evaluation of chitosan–itraconazole co-precipitated nanosuspension for ocular delivery. J. Exp. Nanosci. 2015;10(3):209–221. [Google Scholar]
- 61.Mohanty B, Majumdar D, Mishra S, Panda A, Patnaik S. Development and characterization of itraconazole-loaded solid lipid nanoparticles for ocular delivery. Pharm. Dev. Technol. 2015;20(4):458–464. doi: 10.3109/10837450.2014.882935. [DOI] [PubMed] [Google Scholar]
- 62.Kumar R, Sinha V. Preparation and optimization of voriconazole microemulsion for ocular delivery. Colloids Surf. B Biointerfaces. 2014;117:82–88. doi: 10.1016/j.colsurfb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 63.Kumar R, Sinha V. Solid lipid nanoparticle: an efficient carrier for improved ocular permeation of voriconazole. Drug Dev. Ind. Pharm. 2016;42(12):1956–1967. doi: 10.1080/03639045.2016.1185437. [DOI] [PubMed] [Google Scholar]
- 64.De Sa F, Taveira S, Gelfuso G, Lima E, Gratieri T. Liposomal voriconazole (VOR) formulation for improved ocular delivery. Colloids Surf. B Biointerfaces. 2015;133:331–338. doi: 10.1016/j.colsurfb.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 65.Serrano D, Ruiz-Saldana H, Molero G, Ballesteros M, Torrado J. A novel formulation of solubilised amphotericin B designed for ophthalmic use. Int. J. Pharm. 2012;437(1–2):80–82. doi: 10.1016/j.ijpharm.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 66.Koontz J, Marcy J. Formation of natamycin: cyclodextrin inclusion complexes and their characterization. J. Agric. Food Chem. 2003;51(24):7106–7110. doi: 10.1021/jf030332y. [DOI] [PubMed] [Google Scholar]
- 67.Pahuja P, Kashyap H, Pawar P. Design and evaluation of HP-β-CD based voriconazole formulations for ocular drug delivery. Curr. Drug Deliv. 2014;11(2):223–232. doi: 10.2174/1567201810666131224105205. [DOI] [PubMed] [Google Scholar]
- 68.Gaudana R, Ananthula H, Parenky A, Mitra A. Ocular drug delivery. AAPS J. 2010;12(3):348–360. doi: 10.1208/s12248-010-9183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baldinger J, Doft B, Burns S, Johnson B. Retinal toxicity of amphotericin B in vitrectomised versus non-vitrectomised eyes. Br. J. Ophthalmol. 1986;70:657–661. doi: 10.1136/bjo.70.9.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaji Y, Yamamoto E, Hiraoka T, Oshika T. Toxicities and pharmacokinetics of subconjunctival injection of liposomal amphotericin B. Graefes Arch. Clin. Exp. Ophthalmol. 2008;247(4):549–553. doi: 10.1007/s00417-008-1003-4. [DOI] [PubMed] [Google Scholar]
- 71.Axelrod A, Peyman G, Apple D. Toxicity of intravitreal injection of amphotericin B. Am. J. Ophthalmol. 1973;76(4):578–583. doi: 10.1016/0002-9394(73)90753-8. [DOI] [PubMed] [Google Scholar]
- 72.Cannon J, Fiscella R, Pattharachayakul S, et al. Comparative toxicity and concentrations of intravitreal amphotericin B formulations in a rabbit model. Invest. Ophthalmol. Vis. Sci. 2003;44(5):2112–2117. doi: 10.1167/iovs.02-1020. [DOI] [PubMed] [Google Scholar]
- 73.Qu L, Li L, Xie H. Toxicity and pharmacokinetics of intrastromal injection of amphotericin B in a rabbit model. Curr. Eye Res. 2013;39(4):340–347. doi: 10.3109/02713683.2013.847961. [DOI] [PubMed] [Google Scholar]
- 74.Müller G, Kara-José N, De Castro R. Antifungals in eye infections: drugs and routes of administration. Rev. Bras. Oftalmol. 2013;72(2):132–141. [Google Scholar]
- 75.Thiel M, Zinkernagel A, Burhenne J, Kaufmann C, Haefeli W. Voriconazole concentration in human aqueous humor and plasma during topical or combined topical and systemic administration for fungal keratitis. Antimicrob. Agents Chemother. 2006;51(1):239–244. doi: 10.1128/AAC.00762-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neoh C, Daniell M, Chen S, Stewart K, Kong D. Clinical utility of caspofungin eye drops in fungal keratitis. Int. J. Antimicrob. Agents. 2014;44(2):96–104. doi: 10.1016/j.ijantimicag.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 77.Schulman J, Peyman G, Dietlein J, Fiscella R. Ocular toxicity of experimental intravitreal itraconazole. Int. Ophthalmol. 1991;15(1):21–24. doi: 10.1007/BF00150975. [DOI] [PubMed] [Google Scholar]
- 78.Ahuja M, Dhake A, Sharma S, Majumdar D. Stability studies on aqueous and oily ophthalmic solutions of diclofenac. Yakugaku Zasshi. 2009;129(4):495–502. doi: 10.1248/yakushi.129.495. [DOI] [PubMed] [Google Scholar]
- 79.Ganea E, Harding JJ. Glutathione-related enzymes and the eye. Curr. Eye Res. 2006;31(1):1–11. doi: 10.1080/02713680500477347. [DOI] [PubMed] [Google Scholar]
- 80.Vadlapatla RK, Vadlapudi AD, Pal D, Mitra AK. Role of membrane transporters and metabolizing enzymes in ocular drug delivery. Curr. Drug Metab. 2014;15(7):680–693. doi: 10.2174/1389200215666140926152459. [DOI] [PubMed] [Google Scholar]
- 81.Schwartzman ML, Masferrer J, Dunn MW, Mcgiff JC, Abraham NG. Cytochrome P450, drug metabolizing enzymes and arachidonic acid metabolism in bovine ocular tissues. Curr. Eye Res. 1987;6(4):623–630. doi: 10.3109/02713688709025223. [DOI] [PubMed] [Google Scholar]
- 82.Huang SP, Palla S, Ruzycki P, et al. Aldo-keto reductases in the eye. J. Ophthalmol. 2010:521204. doi: 10.1155/2010/521204. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayman S, Kinoshita JH. Isolation and properties of lens aldose reductase. J. Biol. Chem. 1965;240(2):877–882. [PubMed] [Google Scholar]
- 84.Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006;58(11):1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 85.Yorio T, Clark A, Wax MB. Ocular Therapeutics: Eye On New Discoveries. Academic Press; NY, USA: 2011. General principles and therapeutic targets; pp. 3–94. [Google Scholar]
- 86.Mannermaa E, Vellonen KS, Urtti A. Drug transport in corneal epithelium and blood–retina barrier: emerging role of transporters in ocular pharmacokinetics. Adv. Drug Deliv. Rev. 2006;58(11):1136–1163. doi: 10.1016/j.addr.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 87.Seyfoddin A, Shaw J, Al-Kassas R. Solid lipid nanoparticles for ocular drug delivery. Drug Deliv. 2010;17(7):467–489. doi: 10.3109/10717544.2010.483257. [DOI] [PubMed] [Google Scholar]
- 88.Lindman B, Wennerström H. Micelles. Topics in Chemistry. Vol. 87. Springer; Berlin, Heidelberg: 1980. Micelles. [Google Scholar]
- 89.Zhang K. Ophthalmic Disease Mechanisms and Drug Discovery. World Scientific; Singapore: 2016. [Google Scholar]
- 90.Gupta AK, Madan S, Majumdar DK, Maitra A. Ketorolac entrapped in polymeric micelles: preparation, characterisation and ocular anti-inflammatory studies. Int. J. Pharm. 2000;209(1–2):1–14. doi: 10.1016/s0378-5173(00)00508-1. [DOI] [PubMed] [Google Scholar]
- 91.Liaw J, Chang SF, Hsiao FC. In vivo gene delivery into ocular tissues by eye drops of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO) polymeric micelles. Gene Ther. 2001;8(13):999–1004. doi: 10.1038/sj.gt.3301485. [DOI] [PubMed] [Google Scholar]
- 92.Tong YC, Chang SF, Liu CY, Kao WW, Huang CH, Liaw J. Eye drop delivery of nano-polymeric micelle formulated genes with cornea-specific promoters. J. Gene Med. 2007;9(11):956–966. doi: 10.1002/jgm.1093. [DOI] [PubMed] [Google Scholar]; •• The authors could deliver gene to the anterior segment using polymeric micelles.
- 93.Tong YC, Chang SF, Kao WW, Liu CY, Liaw J. Polymeric micelle gene delivery of bcl-xL via eye drop reduced corneal apoptosis following epithelial debridement. J. Control. Release. 2010;147(1):76–83. doi: 10.1016/j.jconrel.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 94.Hao J, Li SK, Kao WW, Liu CY. Gene delivery to cornea. Brain Res. Bull. 2010;81(2–3):256–261. doi: 10.1016/j.brainresbull.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tu J, Pang H, Yan Z, Li P. Ocular permeability of pirenzepine hydrochloride enhanced by methoxy poly(ethylene glycol)-poly(D, L-lactide) block copolymer. Drug Dev. Ind. Pharm. 2007;33(10):1142–1150. doi: 10.1080/03639040701397381. [DOI] [PubMed] [Google Scholar]
- 96.Di Tommaso C, Torriglia A, Furrer P, Behar-Cohen F, Gurny R, Moller M. Ocular biocompatibility of novel cyclosporin A formulations based on methoxy poly(ethylene glycol)-hexylsubstituted poly(lactide) micelle carriers. Int. J. Pharm. 2011;416(2):515–524. doi: 10.1016/j.ijpharm.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 97.Di Tommaso C, Bourges JL, Valamanesh F, et al. Novel micelle carriers for cyclosporin A topical ocular delivery: in vivo cornea penetration, ocular distribution and efficacy studies. Eur. J. Pharm. Biopharm. 2012;81(2):257–264. doi: 10.1016/j.ejpb.2012.02.014. [DOI] [PubMed] [Google Scholar]; •• The authors have extensively studied delivery of cyclosporine A using polymeric micelles.
- 98.Di Tommaso C, Valamanesh F, Miller F, et al. A novel cyclosporin a aqueous formulation for dry eye treatment: in vitro and in vivo evaluation. Invest. Ophthalmol. Vis. Sci. 2012;53(4):2292–2299. doi: 10.1167/iovs.11-8829. [DOI] [PubMed] [Google Scholar]
- 99.Pepic I, Jalsenjak N, Jalsenjak I. Micellar solutions of triblock copolymer surfactants with pilocarpine. Int. J. Pharm. 2004;272(1–2):57–64. doi: 10.1016/j.ijpharm.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 100.Taha EI, Badran MM, El-Anazi MH, Bayomi MA, El-Bagory IM. Role of Pluronic F127 micelles in enhancing ocular delivery of ciprofloxacin. J. Mol. Liq. 2014;199:251–256. [Google Scholar]
- 101.Alonso MJ, Sanchez A. The potential of chitosan in ocular drug delivery. J. Pharm. Pharmacol. 2003;55(11):1451–1463. doi: 10.1211/0022357022476. [DOI] [PubMed] [Google Scholar]
- 102.Pepic I, Hafner A, Lovric J, Pirkic B, Filipovic-Grcic J. A nonionic surfactant/chitosan micelle system in an innovative eye drop formulation. J. Pharm. Sci. 2010;99(10):4317–4325. doi: 10.1002/jps.22137. [DOI] [PubMed] [Google Scholar]
- 103.Civiale C, Licciardi M, Cavallaro G, Giammona G, Mazzone MG. Polyhydroxyethylaspartamide-based micelles for ocular drug delivery. Int. J. Pharm. 2009;378(1–2):177–186. doi: 10.1016/j.ijpharm.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 104.Usui T, Sugisaki K, Amano S, Jang W-D, Nishiyama N, Kataoka K. New drug delivery for corneal neovascularization using polyion complex micelles. Cornea. 2005;24(8 Suppl.):S39–S42. doi: 10.1097/01.ico.0000178738.29459.59. [DOI] [PubMed] [Google Scholar]
- 105.Benelli U. Systane® lubricant eye drops in the management of ocular dryness. Clin. Ophthalmol. 2011;5:783. doi: 10.2147/OPTH.S13773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yingfang F, Zhuang B, Wang C, Xu X, Xu W, Lv Z. Pimecrolimus micelle exhibits excellent therapeutic effect for Keratoconjunctivitis Sicca. Colloids Surf. B Biointerfaces. 2016;140:1–10. doi: 10.1016/j.colsurfb.2015.11.059. [DOI] [PubMed] [Google Scholar]
- 107.Biswas S, Kumari P, Lakhani PM, Ghosh B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016;83:184–202. doi: 10.1016/j.ejps.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 108.Guo C, Zhang Y, Yang Z, et al. Nanomicelle formulation for topical delivery of cyclosporine A into the cornea: in vitro mechanism and in vivo permeation evaluation. Sci. Rep. 2015;5(1):12968. [Google Scholar]
- 109.Losa C, Calvo P, Castro E, Vila-Jato JL, Alonso MJ. Improvement of ocular penetration of amikacin sulphate by association to poly(butylcyanoacrylate) nanoparticles. J. Pharm. Pharmacol. 1991;43(8):548–552. doi: 10.1111/j.2042-7158.1991.tb03534.x. [DOI] [PubMed] [Google Scholar]
- 110.Musumeci T, Bucolo C, Carbone C, Pignatello R, Drago F, Puglisi G. Polymeric nanoparticles augment the ocular hypotensive effect of melatonin in rabbits. Int. J. Pharm. 2013;440(2):135–140. doi: 10.1016/j.ijpharm.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 111.Warsi MH, Anwar M, Garg V, et al. Dorzolamide-loaded PLGA/vitamin E TPGS nanoparticles for glaucoma therapy: pharmacoscintigraphy study and evaluation of extended ocular hypotensive effect in rabbits. Colloids Surf. B Biointerfaces. 2014;122(122):423–431. doi: 10.1016/j.colsurfb.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 112.Tommaso CD, Bourges JL, Valamanesh F. Novel micelle carriers for cyclosporin A topical ocular delivery: in vivo cornea penetration, ocular distribution and efficacy studies. Eur. J. Pharm. Biopharm. 2012;81(2):257–264. doi: 10.1016/j.ejpb.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 113.Tommaso CD, Torriglia A, Furrer P. Ocular biocompatibility of novel cyclosporin A formulations based on methoxy poly (ethylene glycol)-hexylsubstituted poly (lactide) micelle carriers. Int. J. Pharm. 2011 doi: 10.1016/j.ijpharm.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 114.Tommaso CD, Valamanesh F, Miller F. A novel cyclosporin A aqueous formulation for dry eye treatment: in vitro and in vivo evaluation novel cyclosporin A aqueous formulation for dry eye. Invest. Ophthalmol. Vis. Sci. 2012;53(4):2292–2299. doi: 10.1167/iovs.11-8829. [DOI] [PubMed] [Google Scholar]
- 115.Di Colo G, Zambito Y, Burgalassi S, Nardini I, Saettone MF. Effect of chitosan and of N-carboxymethylchitosan on intraocular penetration of topically applied ofloxacin. Int. J. Pharm. 2004;273(1–2):37–44. doi: 10.1016/j.ijpharm.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 116.Li J, Li Z, Zhou T, et al. Positively charged micelles based on a triblock copolymer demonstrate enhanced corneal penetration. Int. J. Nanomedicine. 2015;10:6027–6037. doi: 10.2147/IJN.S90347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ibrahim SA, Li SK. Efficiency of fatty acids as chemical penetration enhancers: mechanisms and structure enhancement relationship. Pharm. Res. 2010;27(1):115–125. doi: 10.1007/s11095-009-9985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12(1):62–76. doi: 10.1208/s12249-010-9563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schaeffer HE, Krohn DL. Liposomes in topical drug delivery. Invest. Ophthalmol. Vis. Sci. 1982;22(2):220–227. [PubMed] [Google Scholar]
- 120.Meisner D, Pringle J, Mezei M. Liposomal ophthalmic drug delivery. III. Pharmacodynamic and biodisposition studies of atropine. Int. J. Pharm. 1989;55(2):105–113. [Google Scholar]
- 121.Singh K, Mezei M. Liposomal ophthalmic drug delivery system I. Triamcinolone acetonide. Int. J. Pharm. 1983;16(3):339–344. [Google Scholar]; •• The authors have studied effect of liposome on permeation of hydrophilic and hydrophobic molecule and acquired some interesting results.
- 122.Singh K, Mezei M. Liposomal ophthalmic drug delivery system. II. Dihydrostreptomycin sulfate. Int. J. Pharm. 1984;19(3):263–269. [Google Scholar]; •• The authors have studied effect of liposome on permeation of hydrophilic and hydrophobic molecule and acquired some interesting results.
- 123.Yu S, Wang QM, Wang X, et al. Liposome incorporated ion sensitive in situ gels for opthalmic delivery of timolol maleate. Int. J. Pharm. 2015;480(1–2):128–136. doi: 10.1016/j.ijpharm.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 124.Abdelbary G. Ocular ciprofloxacin hydrochloride mucoadhesive chitosan-coated liposomes. Pharm. Dev. Technol. 2011;16(1):44–56. doi: 10.3109/10837450903479988. [DOI] [PubMed] [Google Scholar]
- 125.Kumar R, Sinha VR. Solid lipid nanoparticle: an efficient carrier for improved ocular permeation of voriconazole. Drug Dev. Ind. Pharm. 2016;42(12):1956–1967. doi: 10.1080/03639045.2016.1185437. [DOI] [PubMed] [Google Scholar]
- 126.Kalam A, Sultana Y, Ali A, et al. Part II: enhancement of transcorneal delivery of gatifloxacin by solid lipid nanoparticles in comparison to commercial aqueous eye drops. J. Biomed. Mater. Res. A. 2013;101(6):1828–1836. doi: 10.1002/jbm.a.34467. [DOI] [PubMed] [Google Scholar]
- 127.Liu Z, Zhang X, Wu H, et al. Preparation and evaluation of solid lipid nanoparticles of baicalin for ocular drug delivery system in vitro and in vivo . Drug Dev. Ind. Pharm. 2011;37(4):475–481. doi: 10.3109/03639045.2010.522193. [DOI] [PubMed] [Google Scholar]
- 128.Wang F, Chen L, Zhang D, et al. Methazolamide-loaded solid lipid nanoparticles modified with low-molecular weight chitosan for the treatment of glaucoma: vitro and vivo study. J. Drug Target. 2014;22(9):849–858. doi: 10.3109/1061186X.2014.939983. [DOI] [PubMed] [Google Scholar]
- 129.Li X, Nie SF, Kong J, Li N, Ju CY, Pan WS. A controlled-release ocular delivery system for ibuprofen based on nanostructured lipid carriers. Int. J. Pharm. 2008;363(1–2):177–182. doi: 10.1016/j.ijpharm.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 130.Mehnert W, Mader K. Solid lipid nanoparticles: production, characterization and applications. Adv. Drug Deliv. Rev. 2001;47(2–3):165–196. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 131.Sawant KK, Dodiya SS. Recent advances and patents on solid lipid nanoparticles. Recent Pat. Drug Deliv. Formul. 2008;2(2):120–135. doi: 10.2174/187221108784534081. [DOI] [PubMed] [Google Scholar]
- 132.Azhar Shekoufeh Bahari L, Hamishehkar H. The impact of variables on particle size of solid lipid nanoparticles and nanostructured lipid carriers; a comparative literature review. Adv. Pharm. Bull. 2016;6(2):143–151. doi: 10.15171/apb.2016.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baig MS, Ahad A, Aslam M, Imam SS, Aqil M, Ali A. Application of Box-Behnken design for preparation of levofloxacin-loaded stearic acid solid lipid nanoparticles for ocular delivery: optimization, in vitro release, ocular tolerance, and antibacterial activity. Int. J. Biol. Macromol. 2016;85:258–270. doi: 10.1016/j.ijbiomac.2015.12.077. [DOI] [PubMed] [Google Scholar]
- 134.Balguri SP, Adelli GR, Majumdar S. Topical ophthalmic lipid nanoparticle formulations (SLN, NLC) of indomethacin for delivery to the posterior segment ocular tissues. Eur. J. Pharm. Biopharm. 2016;109:224–235. doi: 10.1016/j.ejpb.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The authors have studied ocular biodistribution of solid lipid nanoparticles and nanostructured lipid carriers (NLC) in the rabbits.
- 135.Basaran E, Demirel M, Sirmagul B, Yazan Y. Cyclosporine-A incorporated cationic solid lipid nanoparticles for ocular delivery. J. Microencapsul. 2010;27(1):37–47. doi: 10.3109/02652040902846883. [DOI] [PubMed] [Google Scholar]
- 136.Beloqui A, Solinis MA, Rodriguez-Gascon A, Almeida AJ, Preat V. Nanostructured lipid carriers: promising drug delivery systems for future clinics. Nanomedicine. 2016;12(1):143–161. doi: 10.1016/j.nano.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 137.Gokce EH, Sandri G, Bonferoni MC, et al. Cyclosporine A loaded SLNs: evaluation of cellular uptake and corneal cytotoxicity. Int. J. Pharm. 2008;364(1):76–86. doi: 10.1016/j.ijpharm.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 138.Hippalgaonkar K, Adelli GR, Hippalgaonkar K, Repka MA, Majumdar S. Indomethacin-loaded solid lipid nanoparticles for ocular delivery: development, characterization, and in vitro evaluation. J. Ocul. Pharmacol. Ther. 2013;29(2):216–228. doi: 10.1089/jop.2012.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hu FQ, Jiang SP, Du YZ, Yuan H, Ye YQ, Zeng S. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf. B Biointerfaces. 2005;45(3–4):167–173. doi: 10.1016/j.colsurfb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 140.Kakkar S, Karuppayil SM, Raut JS, et al. Lipid-polyethylene glycol based nano-ocular formulation of ketoconazole. Int. J. Pharm. 2015;495(1):276–289. doi: 10.1016/j.ijpharm.2015.08.088. [DOI] [PubMed] [Google Scholar]
- 141.Kalam MA, Sultana Y, Ali A, Aqil M, Mishra AK, Chuttani K. Preparation, characterization, and evaluation of gatifloxacin loaded solid lipid nanoparticles as colloidal ocular drug delivery system. J. Drug Target. 2010;18(3):191–204. doi: 10.3109/10611860903338462. [DOI] [PubMed] [Google Scholar]
- 142.Bhagurkar AM, Repka MA, Murthy SN. A novel approach for the development of a nanostructured lipid carrier formulation by hot-melt extrusion technology. J. Pharm. Sci. 2017;106(4):1085–1091. doi: 10.1016/j.xphs.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 143.Cavalli R, Gasco MR, Chetoni P, Burgalassi S, Saettone MF. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int. J. Pharm. 2002;238(1):241–245. doi: 10.1016/s0378-5173(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 144.Sandri G, Bonferoni MC, Gokce EH, et al. Chitosan-associated SLN: in vitro and ex vivo characterization of cyclosporine A loaded ophthalmic systems. J. Microencapsul. 2010;27(8):735–746. doi: 10.3109/02652048.2010.517854. [DOI] [PubMed] [Google Scholar]
- 145.Kumar R, Sinha VR. Lipid nanocarrier: an efficient approach towards ocular delivery of hydrophilic drug (Valacyclovir) AAPS PharmSciTech. 2017;18(3):884–894. doi: 10.1208/s12249-016-0575-2. [DOI] [PubMed] [Google Scholar]
- 146.Sharma AK, Sahoo PK, Majumdar DK, Sharma N, Sharma RK, Kumar A. Fabrication and evaluation of lipid nanoparticulates for ocular delivery of a COX-2 inhibitor. Drug Deliv. 2016;23(9):3364–3373. doi: 10.1080/10717544.2016.1183720. [DOI] [PubMed] [Google Scholar]
- 147.Shen J, Deng Y, Jin X, Ping Q, Su Z, Li L. Thiolated nanostructured lipid carriers as a potential ocular drug delivery system for cyclosporine A: improving in vivo ocular distribution. Int. J. Pharm. 2010;402(1):248–253. doi: 10.1016/j.ijpharm.2010.10.008. [DOI] [PubMed] [Google Scholar]; •• Compares ocular distribution of NLC and surface-modified NLC (thiolation) in rabbits.
- 148.Balguri SP, Adelli GR, Janga KY, Bhagav P, Majumdar S. Ocular disposition of ciprofloxacin from topical, PEGylated nanostructured lipid carriers: effect of molecular weight and density of poly (ethylene) glycol. Int. J. Pharm. 2017;529(1–2):32–43. doi: 10.1016/j.ijpharm.2017.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu R, Wang S, Sun L, et al. A novel cationic nanostructured lipid carrier for improvement of ocular bioavailability: design, optimization, in vitro and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2016;33:28–36. [Google Scholar]
- 150.Huang J, Peng T, Li Y, et al. Ocular cubosome drug delivery system for timolol maleate: preparation, characterization, cytotoxicity, ex vivo, and in vivo evaluation. AAPS PharmSciTech. 2017;18(8):2919–2926. doi: 10.1208/s12249-017-0763-8. [DOI] [PubMed] [Google Scholar]
- 151.Karami Z, Hamidi M. Cubosomes: remarkable drug delivery potential. Drug Discov. Today. 2016;21(5):789–801. doi: 10.1016/j.drudis.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 152.Li J, Wu L, Wu W, et al. A potential carrier based on liquid crystal nanoparticles for ophthalmic delivery of pilocarpine nitrate. Int. J. Pharm. 2013;455(1–2):75–84. doi: 10.1016/j.ijpharm.2013.07.057. [DOI] [PubMed] [Google Scholar]
- 153.Nagarwal RC, Kant S, Singh PN, Maiti P, Pandit JK. Polymeric nanoparticulate system: a potential approach for ocular drug delivery. J. Control. Rel. 2009;136(1):2–13. doi: 10.1016/j.jconrel.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 154.Doktorovova S, Kovacevic AB, Garcia ML, Souto EB. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016;108:235–252. doi: 10.1016/j.ejpb.2016.08.001. [DOI] [PubMed] [Google Scholar]