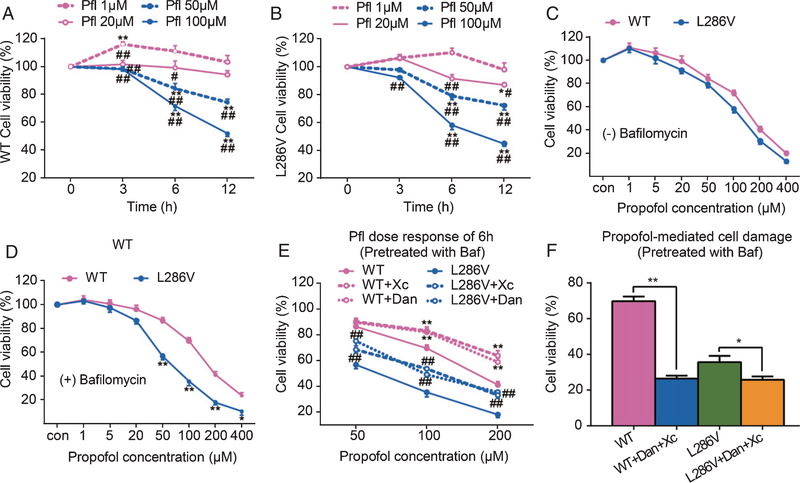
Fig. 1.
Propofol affects cell viability in autophagy activity-dependent manner via activation of InsP3 (InsP3R) or ryanodine (RYR) receptors. PC12 rat pheochromocytoma cells transfected with knocked in point mutation of presenilin 1 (L286V) or wild type (WT) controls were exposed to different concentrations of propofol (1 to 400 μM) for 3, 6, or 12 h. The Cell Counting Kit-8 (CCK-8) was used to determine cell viability. Propofol dose- and time-dependently affect cell viability in both types of cells equally, with low dose (1 μM) for up to 6 h trended to increase cell viability but high concentrations (over 50 μM) significantly reduced cell viability (A and B). *p < 0.01 and **p < 0.001 compared to the beginning time point at each propofol dose group (N ≥ 3). #p < 0.01 and ##p < 0.001 compared to propofol (1 μM) group at each time point (N ≥ 3). Impairment of autophagy flux by bafilomycin did not worsen the 6 h propofol mediated cell damage in WT cells (C), but significantly increased vulnerability of L286V cells to propofol dose-dependent toxicity (D). *p < 0.01 and **p < 0.001 compared to WT (N ≥ 3). The propofol toxicity in autophagy-disrupted L286V cells was inhibited by antagonists for InsP3Rs (E, xestospongin C, Xc 1 μM) or RYRs (E, dantrolene, 30 μM), but paradoxically worsened by the combined use of Xc and dantrolene (F). *p < 0.05 and **p < 0.01 (N ≥ 3). All data in the figure are expressed as the mean ± SEM from at least three separate experiments with triplicates and analyzed by two-way ANOVA followed by Tukey post multiple comparison tests.