Abstract
Purpose
Treatment outcomes and direct medical costs were examined, from a US health payer perspective, of monotherapy with sarilumab 200 mg subcutaneous (SC) every 2 weeks (Q2W) vs adalimumab 40 mg SC Q2W/QW in adult patients with moderately to severely active rheumatoid arthritis who are intolerant of, inadequate responders to, or considered inappropriate candidates for continued methotrexate treatment.
Patients and methods
Short-term analysis was based on 24-week wholesale acquisition costs of drugs and treatment response observed in the MONARCH Phase III trial (NCT02332590) per American College of Rheumatology (ACR) 20/50 criteria and European League Against Rheumatism (EULAR) Moderate/Good Disease Activity Score 28-joint count erythrocyte sedimentation rate. Long-term analysis, which also considered drug administration and routine care costs, was conducted via a 6-month decision tree and a 1- to 10-year Markov model with microsimulation of patient profiles from the MOBILITY Phase III trial (NCT01061736). Utilities and quality-adjusted life-years (QALYs) were estimated by mapping 6-month ACR levels to a relative change in Health Assessment Questionnaire – Disability Index score and via published algorithms.
Results
For sarilumab and adalimumab, respectively, 24-week drug costs were $18,954 and $29,232, and costs per responder were $26,435 vs $50,055 on ACR20; $41,475 vs $98,425 on ACR50; and $22,511 vs $41,230 on EULAR Moderate/Good. Base case results at 10 years for total costs and QALYs were $176,977 and 2.75 for sarilumab and $212,136 and 2.61 for adalimumab, respectively. Sarilumab was consistently the more effective and cost-saving treatment across all short-term and long-term incremental analyses.
Conclusion
Sarilumab monotherapy was the economically dominant treatment on incremental cost per responder and incremental cost per QALY compared with adalimumab monotherapy. These results were maintained within the sensitivity analyses.
Keywords: treatment costs, disease-modifying anti-rheumatic drug, IL-6 inhibitor, rheumatoid arthritis, cost per responser
Introduction
The addition of a targeted disease-modifying antirheumatic drug (DMARD), including either a biologic DMARD (bDMARD) or a targeted synthetic DMARD (tsDMARD), is recommended in treatment guidelines for reaching therapeutic goals in patients with rheumatoid arthritis (RA) who inadequately respond to first-line conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), eg, methotrexate.1,2 However, primarily due to intolerance or contraindication of one or more csD-MARDs,3–7 targeted treatment without the continued use of csDMARDs remains a prevalent practice; real-world data indicate that between 25% and 45% of patients take monotherapy with the targeted treatment regimen rather than combination therapy.3–7 For these patients, a range of monotherapy options are available,8 including the tumor necrosis factor inhibitors adalimumab,9 etanercept,10,11 and certolizumab;12 the T-cell inhibitor abatacept;13 the Janus kinase inhibitor tofacitinib,14 and the anti-IL-6 receptors tocilizumab15,16 and sarilumab.17
Sarilumab is a human monoclonal antibody directed against the IL-6 receptor alpha, inhibiting IL-6-mediated signal transduction. Its efficacy and safety in the treatment of moderately or severely active RA were evaluated in the MONARCH study (NCT02332590),17 which showed equivalent safety and superior efficacy of monotherapy with sarilumab 200 mg subcutaneous (SC) plus placebo every 2 weeks (Q2W) vs adalimumab 40 mg SC plus placebo Q2W/every week (QW) in patients with RA who were intolerant of, inadequate responders to, or considered inappropriate candidates for continued treatment with methotrexate.
To inform clinical and budgetary decisions, evidence of the cost consequences associated with achieving the clinical benefits of this treatment as a monotherapy option for RA patients with moderate-to-severely active RA may be considered by clinicians and payers. By evaluating the drug costs associated with obtaining treatment responses as observed in a trial population, the treatment value of sarilumab relative to a comparator such as adalimumab, which is currently the most commonly used biologic for the treatment of RA in the USA,18 can be considered from a simple, yet robust, perspective.
Objective
This study examined treatment outcomes and direct medical costs associated with treatment using sarilumab compared with adalimumab in adult patients with moderately to severely active RA in the USA. Outcomes were based on treatment responses observed in the MONARCH randomized controlled trial (RCT), which were then extrapolated via long-term simulations over 1- to 10-year time horizons.
Patients and methods
This evaluation of sarilumab compared with adalimumab from a US commercial health care payer perspective was conducted in a target population of patients with moderately or severely active RA who were intolerant of, inadequate responders to, or considered inappropriate candidates for continued methotrexate treatment. The base case analysis was conducted from a short-term perspective to estimate the cost per responder at 24 weeks of treatment. In addition, long-term analyses were conducted to extrapolate the base case results over longer time horizons; deterministic analyses were conducted on the outcome of incremental cost per quality-adjusted life-years (QALYs) at 24 weeks, and 1, 5, and 10 years.
Base case analysis
The base case analysis evaluated drug costs in relation to treatment response rates at 24 weeks.17 Treatment response was defined using three separate endpoints: American College of Rheumatology (ACR) 20 criteria, ACR50 criteria, or European League Against Rheumatism (EULAR) Moderate/Good Disease Activity Score 28-joint count erythrocyte sedimentation rate (DAS28-ESR). Drug costs comprised 2017 US wholesale acquisition costs (WACs) for 24 weeks of treatment with sarilumab 200 mg SC Q2W vs adalimumab 40 mg SC Q2W/QW. For each endpoint, estimates were calculated for the number needed-to-treat (NNT; one/absolute increase in responders). The basis of evaluation was the incremental cost per responder, ie, the difference in 24-week drug cost multiplied by the NNT on each outcome for sarilumab compared with adalimumab.
To account for uncertainty of the base case cost evaluation, two inputs were tested via two sensitivity analyses. First, the OR of the response rate for sarilumab over adalimumab on the three outcomes was reduced to the lower bound of their 95% CIs. The drug cost of adalimumab was then separately varied to account for the 8.2% of patients who required adalimumab dose escalation at week 16 and the 0.5% of patients who required adalimumab dose escalation at week 20 in the trial (consistent with the trial protocol which permitted dose escalation to QW for adalimumab or matching placebo in the sarilumab group for patients who did not achieve ≥20% improvement in tender and swollen joint counts for two consecutive visits).17
Base case analysis population
The intention-to-treat (ITT) population from MONARCH included 184 and 185 patients in the sarilumab and adalim-umab arms, respectively (Table 1). Baseline demographics, including baseline Health Assessment Questionnaire – Disability Index (HAQ-DI) and DAS28-ESR scores, between the two treatment groups were comparable; however, disease duration was longer for the sarilumab group vs the adalimumab group (mean years, [SD]: 8.1 [8.1] vs 6.6 [7.8], respectively; Table 1).17
Table 1.
Demographics/characteristics of MONARCH sarilumab and adalimumab patient populations (base case analysis) and MOBILITY aggregate patient population (individual patient simulation)
| Demographics/characteristics | MONARCH | |
|---|---|---|
| Sarilumab SC 200 mg Q2W (n=184) | Adalimumab SC 40 mg Q2W/QW (n=185) | |
| Age, years, mean ± SD | 50.9±12.6 | 53.6±11.9 |
| Female, n (%) | 157 (85.3) | 150 (81.1) |
| Race, White–Caucasian, n (%) | 171 (92.9) | 164 (88.6) |
| Baseline HAQ-DI, mean ± SD | 1.6±0.6 | 1.6±0.6 |
| Baseline DAS28-ESR, mean ± SD | 6.8±0.8 | 6.8±0.8 |
| Duration of RA, years, mean ± SD | 8.1±8.1 | 6.6±7.8 |
| MOBILITY | ||
| Age, years, mean (range) ± SD | 50.6 (18–75)±11.6 | |
| Female, n (%) | 977 (81.6) | |
| Race, White–Caucasian, n (%) | 1,031 (86.1) | |
| Baseline HAQ-DI, mean (range) ± SD | 1.6 (0.0–3.0)±0.6 | |
| Duration of RA, years, mean (range) ± SD | 9.0 (0.3–44.7)±7.9 | |
Abbreviations: DAS28-ESR, Disease Activity Score 28-joint count erythrocyte sedimentation rate; HAQ-DI, Health Assessment Questionnaire-Disability Index; Q2W, every 2 weeks; QW, once weekly; RA, rheumatoid arthritis; SC, subcutaneous.
Long-term analysis
To extrapolate the base case results over longer time horizons, further analyses were conducted across 1-, 5-, and 10-year time horizons. The extended time horizons considered that, in the long-term, patients’ cycle through multiple lines of targeted DMARDs which have effects on RA outcomes, including health-related quality of life (HRQoL). In addition, the long-term economic implications of the treatments, beyond those of drug costs, were considered. Therefore, the relative impact of additional lines of therapy on HRQoL in terms of QALYs and the wider costs of treatment (eg, routine care) were considered in this long-term perspective.
Model structure
A 6-month decision tree followed by a Markov state transition model spanning from year 1 to 10 was developed in Microsoft Excel® (version 2013). The decision tree applied the initial 24-week efficacy data from MONARCH,17 and then assigned patients to one of three classifications at the end of the 6-month cycle:
Responder: adequate response (ACR20 achieved [inclusive of patients with ACR20, ACR50, and ACR70 responses]) and continuation of initial treatment until discontinuation.
Nonresponder: inadequate response (ACR20 not achieved) and switching to a subsequent treatment line (ie, next bDMARD or csDMARD palliative treatment).
Death.
Following the initial 6-month cycle of the decision tree, all surviving patients could transition to one of the following states in subsequent 6-month intervals of the Markov model:
remain on initial line of treatment,
move to a subsequent line of targeted treatment (for relevant sequences),
move to csDMARD palliative treatment,
Die.
When patients moved to the subsequent treatment, outcomes were evaluated by employing the results of a network meta-analysis of targeted DMARD monotherapies for the treatment of RA (Table 2), which included etanercept, tofacitinib, tocilizumab, and certolizumab. The probability of moving to the next line of treatment was also informed by duration of treatment (ie, time until treatment discontinuation). Treatment discontinuation was based on a de novo analysis of the Canadian RHUMADATA registry (http://rhumadata.info/) based on parametric models fitted to data on time to treatment discontinuation. Of the models (eg, Gompertz, generalized gamma, and log-normal) that indicated the best fit after consideration of Akaike information criterion and Bayesian information criterion, the Gompertz distribution was selected based on visual comparison of the observed and predicted curves and probability plots.
Table 2.
Treatment response rates applied to short-term and long-term models
| Treatment | Treatment response based on: | |||
|---|---|---|---|---|
| ACR20 | ACR50 | ACR70 | EULAR DAS28-ESR Good/Moderate | |
| Comparators | ||||
| Sarilumab 200 mg SC Q2W – response ratesa | 71.7% | 45.7% | 23.4% | 84.2% |
| Adalimumab 40 mg SC Q2W – response ratesa | 58.4% | 29.7% | 11.9% | 70.9% |
| Etanercept 25 mg SC bid QW – response ratesb | ||||
| OR sarilumab vs etanercept | 1.01 | 0.94 | 0.47 | – |
| Derived response rates | 71.5% | 47.2% | 39.4% | – |
| Tofacitinib 5 mg bid oral – response ratesb | ||||
| OR sarilumab vs tofacitinib | 3.11 | 2.42 | 5.1 | – |
| Derived response rates | 44.9% | 25.8% | 5.7% | – |
Notes:
Data from Burmester et al.17
Based on a network meta-analysis of targeted DMARD monotherapies for the treatment of rheumatoid arthritis.
Abbreviations: ACR, American College of Rheumatology; bid, twice daily; DAS28-ESR, Disease Activity Score 28-joint count erythrocyte sedimentation rate; DMARD, disease-modifying antirheumatic agent; EULAR, European League against Rheumatism; Q2W, every 2 weeks; QW, once weekly; SC, subcutaneous.
Patient simulation
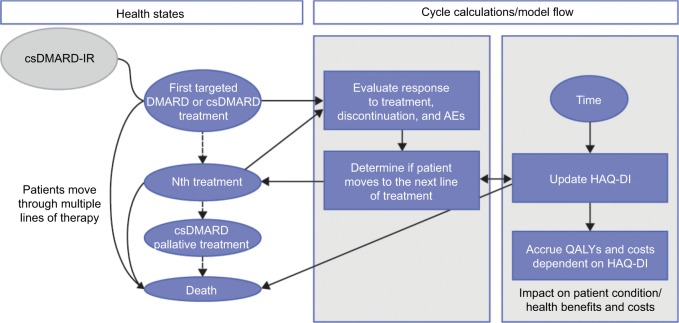
Given the heterogeneity of RA patients,19 expected patient outcomes and costs were estimated via the approach of individual patient simulation (IPS), which tracked patient characteristics, including HAQ-DI scores, over the horizon of the long-term model (Figure 1). Patients simulated through the model were based on individual profiles of patients enrolled in MOBILITY, a pivotal Phase III trial of sarilumab SC in combination with methotrexate.20 Duplicated patients from the MOBILITY ITT population were assigned to each of the treatment arms of the Markov model, ensuring the same characteristics between the patient populations in each arm. Each of these duplicates was then separately tracked through the entirety of the model. This approach ensured that the treatment outcomes were not influenced by baseline differences in patient characteristics in the treatment arms.
Figure 1.
Model structure for long-term analysis.
Abbreviations: AE, adverse event; csDMARD, conventional synthetic disease-modifying antirheumatic drug; csDMARD-IR, inappropriate response or intolerance to csDMARDs/methotrexate; HAQ-DI, Health Assessment Questionnaire Disability Index; QALY, quality-adjusted life-year.
When patients switched DMARDs, it was assumed that their response to the present treatment was not dependent upon the line in which the treatment was administered (eg, first-, second-, or third-line in the treatment sequence). This approach was consistent with previously published cost-effectiveness models in RA. In addition, patients on the terminal, palliative treatment with csDMARDs were assumed to achieve no response on ACR20.
For each cohort, ACR response was mapped to a relative change in HAQ-DI score, a measure of physical function in patients with RA.21 Expected percentage changes in HAQ-DI scores for sarilumab and adalimumab were based on response data from the MOBILITY trial,17 with the mapping conducted via an algorithm estimated from the trial data. Changes in HAQ-DI from baseline to week 24 were then predicted for each patient, within each of the ACR response categories (ie, ACR70, ACR50, ACR20, or no ACR response). HAQ-DI changes associated with the response levels were not treatment-specific and, while the patient was on a targeted treatment, the HAQ-DI score was assumed to remain constant.22 Conversely, HAQ-DI scores for patients on csDMARD palliative treatment were assumed to increase (worsen) annually by 0.045.23 When patients discontinued treatment, their HAQ-DI scores returned to their baseline HAQ-DI22 and then followed the trend in scores for the line of treatment into which they transitioned. The change in HAQ-DI score at the initiation and discontinuation of a treatment is typically gradual; therefore, to capture this gradual change a midway HAQ-DI value in the cycle of the treatment switch was modeled.
IPS population
In MOBILITY, 1,197 adult patients fulfilling the 1987 ACR classification criteria for RA24,25 with moderate-to-severe RA were randomized (1:1:1) to sarilumab SC 150 mg, 200 mg or placebo SC Q2W added to methotrexate. The ages of patients ranged from 18 to 75 years (mean 50.6±11.6); 81.6% were female and 86.1% were White/Caucasian. Duration of RA ranged from 0.3 to 44.7 years (mean 9.0±7.9), and baseline HAQ-DI scores ranged from 0 to 3.0 (mean 1.6±0.6; Table 1).20
Utilities, adverse events, and mortality
As the net measure of long-term RA impairment and treatment benefit, QALYs were estimated on patient life expectancy and utility weights. Utility weights were calculated on HAQ-DI scores for each treatment cycle using an equation fitted on data from several trials for adalimumab and based on HAQ-DI score and sex: utility =0.76–0.28 × HAQ-DI + 0.05 × female.26,27 Adverse events were not separately evaluated in the model but implicit in the utility equations and based on safety data from all relevant trials. Life expectancy, or life-years (LYs) gained, was based on US-specific life tables adjusted according to current HAQ-DI and HAQ-DI increase.28 General population mortality rates for males and females in the USA were obtained from the National Vital Statistics Reports from 2015.29
Treatment costs
Drug costs were based on the February 18, 2018, WAC of each drug applied to the dosing and treatment schedules specified in the prescribing information for the treatment in the sequences (Table 3). For csDMARD palliative treatment, cost was based on the weighted average cost of different csDMARDs according to the real-world rate of use.30 For all drugs, the rate of treatment adherence was assumed to be 100%.
Table 3.
Drug costs
| 6-Monthly drug costs (US$)a | |||
|---|---|---|---|
| Drug | Dose | 6-Monthly induction costs | 6-Monthly maintenance costs |
| Sarilumab | 200 mg SC Q2W | $20,604 | $20,604 |
| Adalimumab | 40 mg SC Q2W | $31,777 | $31,777 |
| Etanercept | 50 mg SC QW | $31,777 | $31,777 |
| Tofacitinib | 5 mg oral bid | $24,932 | $24,932 |
| csDMARD palliative treatmentb | N/A | N/A | $3,820 |
Notes:
Based on wholesale acquisition costs (February 18, 2018). Long-term CE analysis considered 6 months cycle length of 26.08 weeks (=365.25/7); short-term CE analysis considered 24 weeks trial duration (over 52 yearly number of weeks), making treatment costs equal $18,954 and $29,232 for sarilumab and adalimumab, respectively, in this first analysis rather than the figures given in the table.
Based on the following distribution of patients: 12.5% on methotrexate tablet alone, 12.5% on methotrexate syringe alone, 10% on prednisolone alone, 35% on methotrexate + prednisolone, 5% on sulfasalazine, 5% on leflunomide, 5% on hydroxychloroquine, and 15% on no treatment.30
Abbreviations: bid, twice daily; csDMARD, conventional synthetic disease-modifying antirheumatic drug; N/A, not applicable; Q2W, every 2 weeks; QW, once weekly; SC, subcutaneous.
Other direct medical costs comprise disease management or routine care costs, non-DMARD medication use, outpatient service use, and hospitalization,31 with costs adjusted for age, disease duration, comorbidities, HAQ-DI score at baseline, current HAQ-DI score, sex, type of DMARD received, number of previous DMARDs, years of education, and ethnicity.
Long-term sensitivity analyses
The long-term analysis considered three varying treatment sequences following first-line targeted treatment with sarilumab or adalimumab:
csDMARD palliative treatment as second-line treatment (evaluated at 1, 5, and 10 years).
A second-line targeted treatment comprising either etanercept or tofacitinib followed by csDMARD palliative treatment as final treatment (evaluated at 5 and 10 years).
Tofacitinib as a third-line treatment, for the second-line etanercept cohort, followed by csDMARD palliative treatment as final treatment (evaluated at 5 and 10 years).
Deterministic analysis
For the long-term evaluation, total costs, LY gained, and QALYs were calculated, with a standard annual discount rate of 3% applied to both total costs and health outcomes. Evaluation was conducted on incremental cost-effectiveness ratios for sarilumab vs adalimumab in terms of costs and QALYs gained within each sensitivity analysis.
Results
Base case analysis
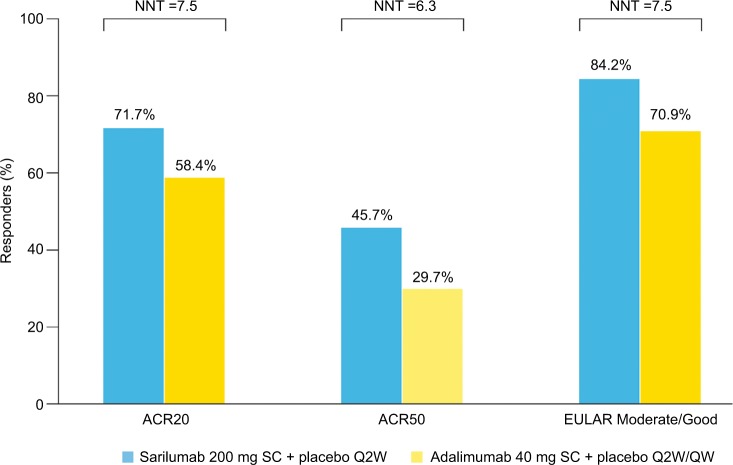
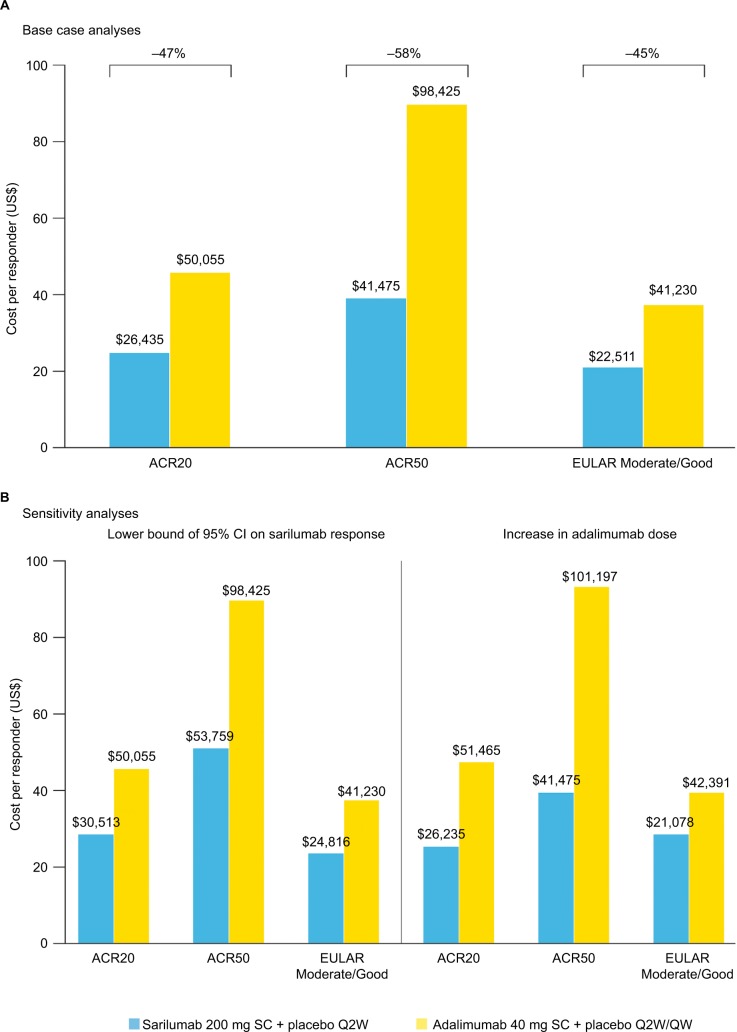
Estimated NNTs for sarilumab vs adalimumab based on ACR20, ACR50, and EULAR Moderate/Good at week 24 were 7.5, 6.3, and 7.5, respectively (Figure 2), and 24-week drug costs were $18,954 for sarilumab 200 mg SC Q2W and $29,232 for adalimumab 40 mg SC Q2W. Based on these outcomes, base case estimates of cost per responder for sarilumab were $26,435, $41,475, and $22,511 vs adalimumab at $50,055, $98,425, and $41,230 using ACR20, ACR50, and EULAR Moderate/Good, respectively. The cost per responder for sarilumab vs adalimumab was 47% lower based on ACR20, 58% lower based on ACR50, and 45% lower based on EULAR Moderate/Good (Figure 3A). Overall, at 24 weeks, sarilumab remained the more effective and cost-saving treatment, also when relative efficacy was varied to the 95% lower bound of the OR CI and adalimumab dose escalation was considered (Figure 3B).
Figure 2.
Responders (%) and NNT.
Abbreviations: ACR, American College of Rheumatology; EULAR, European League Against Rheumatism; NNT, number needed-to-treat; Q2W, every 2 weeks; QW, once weekly; SC, subcutaneous.
Figure 3.
Cost per responder at 24 weeks: (A) base case analysis and (B) sensitivity analyses.
Abbreviations: ACR, American College of Rheumatology; EULAR, European League Against Rheumatism; Q2W, every 2 weeks; QW, once weekly; SC, subcutaneous.
Long-term analyses
1-year horizon
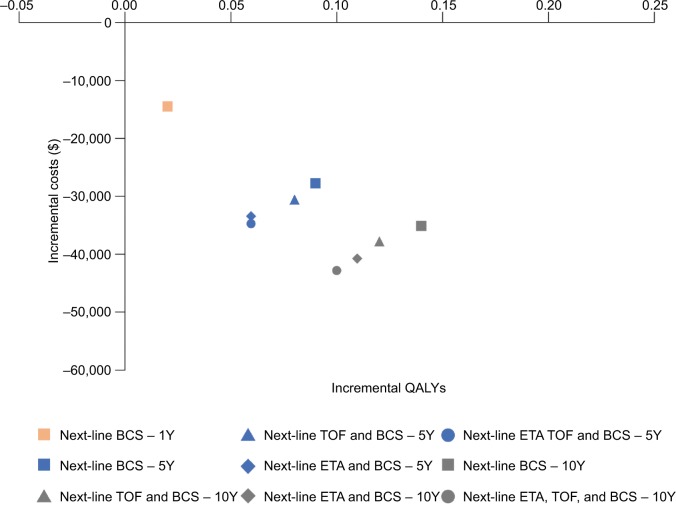
Over a 1-year time model horizon, with subsequent csD-MARD palliative treatment after irresponsiveness with initial treatment, total treatment costs for sarilumab and adalimumab were estimated to be $37,095 and $51,527, respectively (Table 4). Both sarilumab and adalimumab were associated with 0.96 LYs; however, when adjusted for HRQoL, QALYs for sarilumab were 0.02 greater (0.3828 QALYs) than for adalimumab (0.3659 QALYs; Table 5). Lower costs for sarilumab coupled with slightly greater QALYs, resulted in sarilumab, dominating adalimumab in a 1-year horizon in terms of incremental cost per QALY (Figure 4).
Table 4.
Long-term analyses: costs
| Treatment sequence | Time horizon (years) | Sarilumab costs (US$) | Adalimumab costs (US$) | Incremental total costs: sarilumab vs adalimumab | ||||
|---|---|---|---|---|---|---|---|---|
| Drug | Routine | Total | Drug | Routine | Total | |||
| Next line: csDMARD palliative treatment | 1 | $33,056 | $4,039 | $37,095 | $47,378 | $4,150 | $51,527 | −$14,432 |
| 5 | $95,039 | $19,278 | $114,317 | $122,202 | $19,837 | $142,039 | −$27,722 | |
| 10 | $140,728 | $36,248 | $176,977 | $175,063 | $37,073 | $212,136 | −$35,159 | |
| Next lines: tofacitinib > csDMARD palliative treatment | 5 | $133,457 | $18,850 | $152,307 | $163,474 | $19,370 | $182,844 | −$30,537 |
| 10 | $195,620 | $35,776 | $231,396 | $232,544 | $36,568 | $269,112 | −$37,716 | |
| Next lines: etanercept > csDMARD palliative treatment | 5 | $165,186 | $18,384 | $183,570 | $198,083 | $18,849 | $216,931 | −$33,362 |
| 10 | $247,966 | $34,880 | $282,847 | $288,019 | $35,603 | $323,621 | −$40,774 | |
| Next lines: etanercept > tofacitinib > csDMARD palliative treatment | 5 | $188,847 | $18,122 | $206,969 | $223,368 | $18,578 | $241,946 | −$34,977 |
| 10 | $288,454 | $34,471 | $322,925 | $330,455 | $35,186 | $365,640 | −$42,716 | |
Abbreviation: csDMARD, conventional synthetic disease-modifying antirheumatic drug.
Table 5.
Long-term analyses: outcomes
| Treatment sequence | Time horizon (years) | Sarilumab outcomes | Adalimumab outcomes | Incremental QALYs: sarilumab vs adalimumab | ||||
|---|---|---|---|---|---|---|---|---|
| Time on treatment 1 | LYs | QALYs | Time on treatment 1 | LYs | QALYs | |||
| Next line: csDMARD palliative treatment | 1 | 0.77 | 0.96 | 0.38 | 0.72 | 0.96 | 0.37 | 0.02 |
| 5 | 1.93 | 4.38 | 1.69 | 1.66 | 4.38 | 1.60 | 0.09 | |
| 10 | 2.66 | 7.79 | 2.75 | 2.26 | 7.77 | 2.61 | 0.14 | |
| Next lines: tofacitinib > csDMARD palliative treatment | 5 | 1.93 | 4.39 | 1.82 | 1.66 | 4.38 | 1.75 | 0.08 |
| 10 | 2.66 | 7.81 | 3.01 | 2.26 | 7.80 | 2.89 | 0.12 | |
| Next lines: etanercept > csDMARD palliative treatment | 5 | 1.93 | 4.39 | 1.95 | 1.66 | 4.39 | 1.89 | 0.06 |
| 10 | 2.66 | 7.84 | 3.25 | 2.26 | 7.83 | 3.15 | 0.11 | |
| Next lines: etanercept > tofacitinib > csDMARD palliative treatment | 5 | 1.93 | 4.40 | 2.02 | 1.66 | 4.40 | 1.97 | 0.06 |
| 10 | 2.66 | 7.86 | 3.44 | 2.26 | 7.85 | 3.34 | 0.10 | |
Abbreviation: csDMARD, conventional synthetic disease-modifying antirheumatic drug.
Figure 4.
Long-term incremental analyses.
Abbreviations: BCS, best care/palliative treatment; ETA, etanercept; TOF, tofacitinib; QALY, quality-adjusted life-year; Y, year.
5- and 10-year horizons
In the 5- and 10-year model horizons, sarilumab was the dominant treatment option across all sensitivity analyses compared with adalimumab, regardless of whether csDMARD palliative treatment or targeted DMARDs were administered subsequently after first-line treatment (Tables 4 and 5; Figure 4).
When csDMARD palliative treatment was assumed to be the second-line treatment, total costs were $114,317 at 5 years and $176,977 at 10 years for sarilumab and $142,039 at 5 years and $212,136 at 10 years for adalimumab. In this case, sarilumab also provided greater QALYs, 1.69 at 5 years and 2.75 at 10 years vs adalimumab which provided 1.60 QALYs at 5 years and 2.61 QALYs at 10 years.
From 5- and 10-year perspectives with tofacitinib as the second-line treatment, total costs were $152,307 for 1.82 QALYs at 5 years and $231,396 for 3.01 QALYs at 10 years for sarilumab vs $182,844 for 1.75 QALYs at 5 years and $269,112 for 2.89 QALYs at 10 years for adalimumab.
Similarly, lower 5- and 10-year costs and higher QALYs for sarilumab were obtained when etanercept was the second-line treatment, with $183,570 for 1.95 QALYs at 5 years and $282,847 for 3.25 QALYs at 10 years vs $216,931 for 1.89 QALYs at 5 years and $323,621 for 3.15 QALYs at 10 years for adalimumab.
From 5- and 10-year perspectives with two lines of targeted DMARD sequential treatment (etanercept as second-line followed by tofacitinib as third-line), lower 5- and 10-year costs and greater QALYs for sarilumab were sustained: $206,969 for 2.02 QALYs at 5 years and $322,925 for 3.44 QALYs at 10 years vs adalimumab with $241,946 for 1.97 QALYs at 5 years and $365,640 for 3.34 QALYs at 10 years.
Discussion
Evidence from the MONARCH head-to-head RCT indicated that sarilumab monotherapy demonstrated superiority over adalimumab monotherapy by improving the signs, symptoms, and physical functions for patients with moderately or severely active RA who should not continue treatment with methotrexate due to intolerance or inadequate response.17 In the short-term cost analysis evaluating 24-week outcomes in the MONARCH RCT, the higher levels of responses for sarilumab vs adalimumab on ACR20, ACR50, and EULAR Moderate/Good, coupled with lower 24-week drug costs, resulted in sarilumab being the economically dominant treatment option in terms of incremental cost per responder analyses across all endpoints. The favorable outcomes for sarilumab were maintained within the sensitivity analyses, which tested the lower rate of sarilumab response and increased dose of adalimumab.
The clinical and cost benefits of sarilumab were confirmed by examining outcomes from medium- to long-term perspectives of up to 10 years, which considered that patients who do not reach treatment target with an initial monotherapy are then switched to one or more further lines of targeted DMARD monotherapy. Sensitivity analyses were conducted based on either immediate csDMARD palliative care, or bDMARD or tsDMARD administered following inadequate response to the initial treatment (sarilumab or adalimumab) in the sequence. Based on IPS to estimate LYs, QALYs, and costs, the analyses indicated that the incremental value of sarilumab over adalimumab, and its economic dominance in terms of better health outcomes and reduced costs, was sustained even following up to three lines of subsequent targeted DMARDs.
The benefit became further evident as the time horizon of analysis increased, with the relative benefits of treatment durability with sarilumab accruing in the longer time horizons. For example, as the horizon increased, patients who initiated treatment with adalimumab tended to discontinue earlier than sarilumab, thereby accruing more costs associated with subsequent bDMARD treatment lines. The long-term analyses also revealed the gain in utilities for sarilumab as time treatment accrued. This benefit started becoming evident in the 1-year analysis of the sequence of csDMARD palliative treatment as second-line treatment. While sarilumab and adalimumab were both associated with 0.96 LYs, differentiation in life expectancy was not able to accrue within a 1-year horizon, with only a slightly greater improvement in HRQoL becoming evident (0.38 QALYs for sarilumab vs 0.37 for adalimumab). This difference in HRQoL over the long term would have stemmed from the slightly increased time on sarilumab treatment (0.77 years) vs adalimumab (0.72 years) and reflected in the HAQ-DI scores and utilities associated with treatment, with the incremental scores and associated QALYs increasing as the time horizon increased.
Some limitations of the study must be noted. First, the current analysis did not consider price discounts or rebates to the WAC, which would impact total medical costs. In addition, similar to other cost evaluations of DMARDs in RA,32,33 the treatment sequences of this study were independent of previous treatment (ie, all patients moved to the subsequent line in the treatment pathway without considerations of patient preferences and effect of previous treatment on the effectiveness of the next therapy). Furthermore, while the present analysis captured a longer horizon than previously published DMARD treatment cost per outcome studies,32,33 one limitation of a longer analysis scope is that, as more parameters and assumptions are included, more uncertainty is introduced into the analysis. However, in the absence of long-term observational evidence to base the economic evaluation of monotherapy with sarilumab vs adalimumab, simulations of the disease evolution were warranted. Therefore, the present evaluation included long-term analysis based on the assumptions and relationships that are common to previously published and validated RA models. Nonetheless, the reliability of the present long-term analyses is owed to the application of microsimulation using individual patient data from the MOBILITY trial; this modeled the heterogeneity of the RA patient population. While MOBILITY was not a monotherapy trial, it provided a larger dataset than MONARCH, but the patient characteristics between the two trials were comparable.
Reaching treatment targets as early as possible in the RA care journey is critical for both treatment-naïve patients and those already on the treatment pathway.1,2,34 The principle of treating-to-target, in addition to the availability of a wide-ranging armamentarium of targeted DMARDs with different modes of action, has made this increasingly possible.35 For patients who cannot benefit from the continuation of methotrexate in addition to a targeted DMARD, either due to contraindication36 or preference, the prompt therapeutic changes required to reach treatment target is afforded by targeted DMARDs available for use as monotherapies. The present cost evaluation, based on direct RCT evidence and supported with simulated long-term outcomes, has highlighted the clinical and economic value of sarilumab as a monotherapy option for consideration by payers, rheumatologists, and patients.
Conclusion
In the paradigm of targeted DMARD monotherapy for patients with RA, sarilumab compared with adalimumab appears to be an economically dominant treatment option with respect to 24-week outcomes from the MONARCH RCT, with safety consistent with IL-6R blockade. Given the higher levels of responses on ACR20, ACR50, and EULAR Moderate/Good, coupled with the lower drug costs, sarilumab was the favorable treatment in terms of incremental cost per responder across all 24-week analyses; these results were maintained within the sensitivity analyses. In addition, long-term analyses, which considered treatment sequencing and associated outcomes, underscored the value of sarilumab treatment over horizons of 1, 5, and 10 years.
Acknowledgments
Medical writing assistance and editorial support, under the direction of the authors, were, respectively, provided by Gauri Saal, MA Economics, and Sinead Stewart of Prime (Knutsford, UK), funded by Sanofi/Regeneron according to Good Publication Practice guidelines (http://annals.org/aim/article/2424869). The sponsor was involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. The authors had unrestricted access to study data, were responsible for all content and editorial decisions, and received no honoraria related to the development of this publication.
Footnotes
Author contributions
All authors made substantial contributions to conception and design, data acquisition, or data analysis and interpretation; drafting the article or critically revising it for important intellectual content; final approval of the version to be published; and are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Disclosure
AK and CC are current employees of and stockholders in Regeneron Pharmaceuticals, Inc. MF and UGM are employees of and stockholders in Sanofi. CP is a former employee and current stockholder in Sanofi, and a current employee of Novartis. KM has received grant funding from Pfizer and Rheumatology Research Foundation. An abstract and poster of this paper, based on interim study findings, were presented at the 2017 meeting of the American College of Rheumatology/Association of Rheumatology Health Professionals (ACR/ARHP) Annual Meeting in San Diego, CA, USA: https://acrabstracts.org/abstract/sarilumab-for-the-treatment-of-active-moderate-to-severe-rheumatoid-arthritis-ra-an-analysis-of-cost-per-effectively-treated-patient/. The authors report no other conflicts of interest in this work.
References
- 1.Singh JA, Saag KG, Bridges SL, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016;68(1):1–25. doi: 10.1002/acr.22783. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 3.Catay E, Bravo M, Rosa J, Soriano ER. Prevalence of biologics monotherapy in a cohort of patients with rheumatoid arthritis in daily clinical practice. BMC Musculoskelet Disord. 2016;17(1):110. doi: 10.1186/s12891-016-0959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emery P, Sebba A, Huizinga TW. Biologic and oral disease-modifying antirheumatic drug monotherapy in rheumatoid arthritis. Ann Rheum Dis. 2013;72(12):1897–1904. doi: 10.1136/annrheumdis-2013-203485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engel-Nitz NM, Ogale S, Kulakodlu M. Use of anti-tumor necrosis factor monotherapy and adherence with non-biologic disease-modifying anti-rheumatic drugs in combination with anti-tumor necrosis factor therapy among rheumatoid arthritis patients in a real-world setting [abstract. Arthritis Rheum. 2012;69(Suppl 10):378. [Google Scholar]
- 6.Pappas DA, Reed GW, Saunders K, et al. Characteristics associated with biologic monotherapy use in biologic-naive patients with rheumatoid arthritis in a US registry population. Rheumatol Ther. 2015;2(1):85–96. doi: 10.1007/s40744-015-0008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choquette D, Thomas O, Arundine M. Lower than expected levels of DMARD acquisition immediately pre and post biologic initiation in rheumatoid arthritis patients. Arthritis Rheum. 2012;64(Suppl 10):1841. [Google Scholar]
- 8.Detert J, Klaus P. Biologic monotherapy in the treatment of rheumatoid arthritis. Biologics. 2015;9:35–43. doi: 10.2147/BTT.S53361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54(1):26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 10.Curtis JR, Yang S, Chen L, et al. Predicting low disease activity and remission using early treatment response to antitumour necrosis factor therapy in patients with rheumatoid arthritis: exploratory analyses from the TEMPO trial. Ann Rheum Dis. 2012;71(2):206–212. doi: 10.1136/ard.2011.153551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannon GW, Wang BC, Park GS, Koenig A, Collier DH, Keystone EC. Remission in rheumatoid arthritis patients treated with etanercept monotherapy: clinical practice and clinical trial experience. Clin Exp Rheumatol. 2013;31(6):919–925. [PubMed] [Google Scholar]
- 12.Fechtenbaum M, Md Yusof MY, Emery P. Certolizumab pegol in rheumatoid arthritis: current update. Expert Opin Biol Ther. 2014;14(6):841–850. doi: 10.1517/14712598.2014.900043. [DOI] [PubMed] [Google Scholar]
- 13.Nash P, Nayiager S, Genovese MC, et al. Immunogenicity, safety, and efficacy of abatacept administered subcutaneously with or without background methotrexate in patients with rheumatoid arthritis: results from a phase III, international, multicenter, parallel-arm, open-label study. Arthritis Care Res. 2013;65(5):718–728. doi: 10.1002/acr.21876. [DOI] [PubMed] [Google Scholar]
- 14.Lee EB, Fleischmann R, Hall S, et al. ORAL Start Investigators ,Tofacitinib versus methotrexate in rheumatoid arthritis N Engl J Med 2014370252377–2386. [DOI] [PubMed] [Google Scholar]
- 15.Gabay C, Emery P, van Vollenhoven R, et al. ADACTA Study Investigators Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381(9877):1541–1550. doi: 10.1016/S0140-6736(13)60250-0. [DOI] [PubMed] [Google Scholar]
- 16.Nakashima Y, Kondo M, Miyahara H, Iwamoto Y. Drug delivery options to increase patient adherence and satisfaction in the management of rheumatoid arthritis – focus on subcutaneous tocilizumab. Drug Des Devel Ther. 2014;8:913–919. doi: 10.2147/DDDT.S52099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burmester GR, Lin Y, Patel R, et al. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Ann Rheum Dis. 2017;76(5):840–847. doi: 10.1136/annrheumdis-2016-210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rashid N, Lin AT, Aranda G, et al. Rates, factors, reasons, and economic impact associated with switching in rheumatoid arthritis patients newly initiated on biologic disease modifying anti-rheumatic drugs in an integrated healthcare system. J Med Econ. 2016;19(6):568–575. doi: 10.3111/13696998.2016.1142448. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Nasser AM, Rasker JJ, Valkenburg HA. Epidemiological and clinical aspects relating to the variability of rheumatoid arthritis. Semin Arthritis Rheum. 1997;27(2):123–140. doi: 10.1016/s0049-0172(97)80012-1. [DOI] [PubMed] [Google Scholar]
- 20.Genovese MC, Fleischmann R, Kivitz AJ, et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a phase III study. Arthritis Rheu-matol. 2015;67(6):1424–1437. doi: 10.1002/art.39093. [DOI] [PubMed] [Google Scholar]
- 21.Bruce B, Fries JF. The Health Assessment Questionnaire (HAQ) Clin Exp Rheumatol. 2005;23(5 Suppl 39):S14–18. [PubMed] [Google Scholar]
- 22.Stevenson M, Archer R, Tosh J. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation [erratum] Health Technol Assess. 2016;20(35):1–610. doi: 10.3310/hta20350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Institute for Health and Care Excellence Adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis, NICE technology appraisal guidance [TA130]; 2007. [Accessed October 21, 2016]. Available from: https://www.nice.org.uk/guidance/ta130.
- 24.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 25.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 26.Boggs R, Sengupta N, Ashraf T. UT3 estimating health utility from a physical function assessment in rheumatoid arthritis (RA) patients treated with adalimumab (D2E7) Value Health. 2002;5(6):452–453. [Google Scholar]
- 27.Bansback NJ, Brennan A, Ghatnekar O. Cost effectiveness of adalimumab in the treatment of patients with moderate to severe rheumatoid arthritis in Sweden. Ann Rheum Dis. 2005;64(7):995–1002. doi: 10.1136/ard.2004.027565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michaud K, vera-Llonch M, Oster G. Mortality risk by functional status and health-related quality of life in patients with rheumatoid arthritis. J Rheumatol. 2012;39(1):54–59. doi: 10.3899/jrheum.110491. [DOI] [PubMed] [Google Scholar]
- 29.Arias E. United States life tables, 2011. Natl Vital Stat Rep. 2015;64(11):1–63. [PubMed] [Google Scholar]
- 30.Diamantopoulos A, Benucci M, Capri S, et al. Economic evaluation of tocilizumab combination in the treatment of moderate-to-severe rheumatoid arthritis in Italy. J Med Econ. 2012;15(3):576–585. doi: 10.3111/13696998.2012.665110. [DOI] [PubMed] [Google Scholar]
- 31.Wailoo AJ, Bansback N, Brennan A, Michaud K, Nixon RM, Wolfe F. Biologic drugs for rheumatoid arthritis in the Medicare program: a cost-effectiveness analysis. Arthritis Rheum. 2008;58(4):939–946. doi: 10.1002/art.23374. [DOI] [PubMed] [Google Scholar]
- 32.Claxton L, Jenks M, Taylor M, et al. An economic evaluation of tofacitinib treatment in rheumatoid arthritis: modeling the cost of treatment strategies in the United States. J Manag Care Spec Pharm. 2016;22(9):1088–1102. doi: 10.18553/jmcp.2016.22.9.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batticciotto A, Ravasio R, Riva M, Sarzi-Puttini P. Efficacy and treatment costs of monotherapy with bDMARDs in the treatment of rheumatoid arthritis in patients intolerant to or inappropriate to continue treatment with methotrexate. Adv Ther. 2016;33(8):1360–1373. doi: 10.1007/s12325-016-0372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monti S, Montecucco C, Bugatti S, Caporali R. Rheumatoid arthritis treatment: the earlier the better to prevent joint damage. RMD Open. 2015;1(Suppl 1):e000057. doi: 10.1136/rmdopen-2015-000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramiro S, Landewé R, van der Heijde D, Harrison D, Collier D, Michaud K. Discontinuation rates of biologics in patients with rheumatoid arthritis: are TNF inhibitors different from non-TNF inhibitors? RMD Open. 2015;1(1):e000155. doi: 10.1136/rmdopen-2015-000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson WL. Sarilumab monotherapy for RA superior in MONARCH. 2016. [Accessed January 10, 2018]. Available from: http://www.rheumatologynetwork.com/rheumatoid-arthritis/sarilumab-monotherapy-ra-superior-monarch.