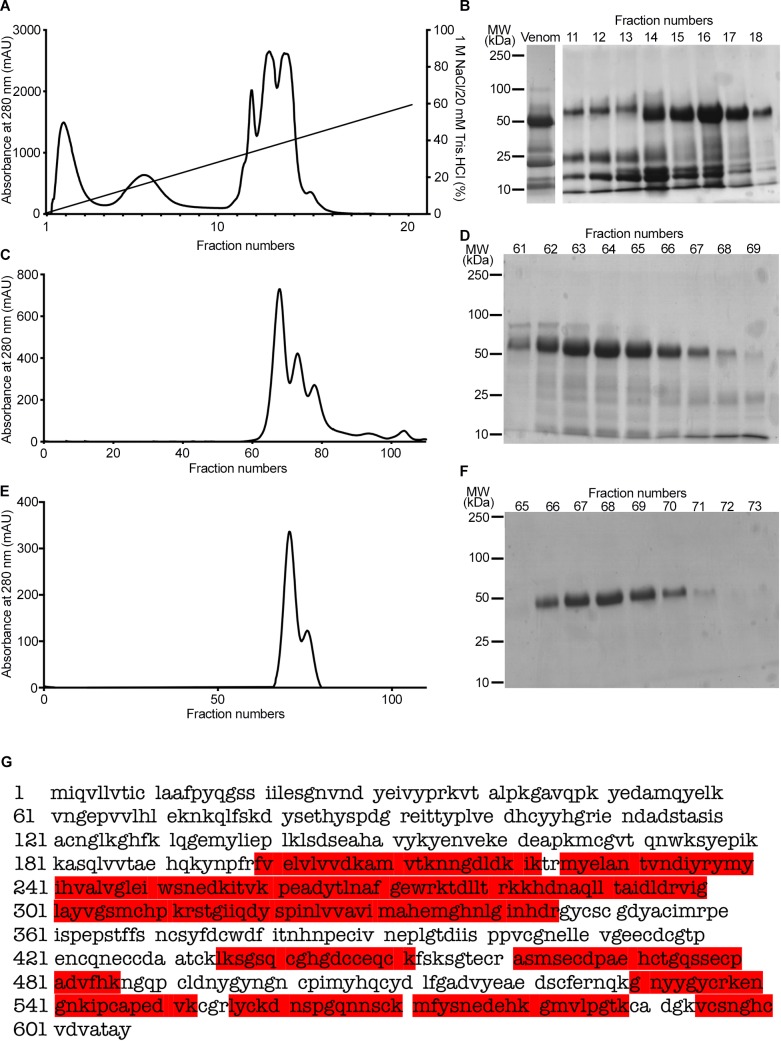
Fig 1. Purification of CAMP from the venom of C. atrox.
A, A chromatogram demonstrates the purification profile of 10mg of whole C. atrox venom by anion exchange chromatography. B, a Coomassie stained gel displays the protein profile of whole C. atrox venom and fractions 11–18 of anion exchange chromatography. A chromatogram (C) and Coomassie stained gel (D) show the purification profile of fractions 14–18 of anion exchange chromatography by gel filtration. E, a chromatogram of the second step of gel filtration for fractions 62–67 from the previous step and (F) a Coomassie stained gel shows the purified protein at approximately 50kDa. G, the tryptic digested samples of the purified protein were analysed by mass spectrometry and the identified peptide sequences match (via Mascot search) with the known sequence of VAP2A (coverage 43%; the mass spectrometry-identified peptide sequences of the purified protein are shown in red) and confirms that the purified protein is most likely to be VAP, a group III metalloprotease. The purified protein was named as CAMP to represent C. atrox metalloprotease.