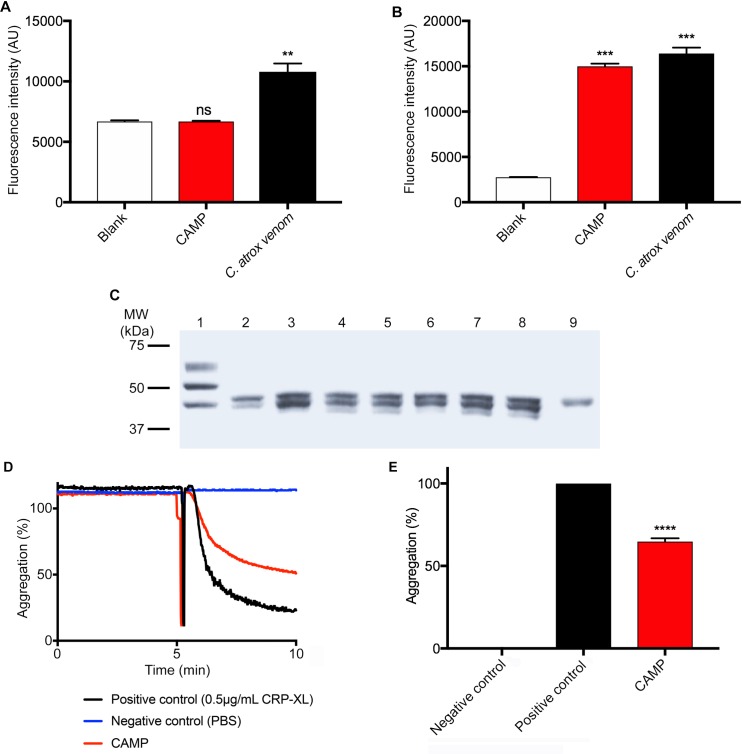
Fig 2. The functional characterisation of CAMP.
A, the serine protease activity of 10μg/mL whole venom or CAMP was analysed using a fluorogenic substrate, Nα-Benzoyl-L-Arginine-7-Amido-4-methylcoumarin hydrochloride (BAAMC) by spectrofluorimetry. Similarly, (B) the metalloprotease activity of 10μg/mL whole venom or CAMP was analysed using DQ-gelatin, a specific fluorogenic substrate for collagenolytic enzymes and the level of fluorescence was measured by spectrofluorimetry. C, a Coomassie stained gel demonstrates the fibrinogenolytic activity of CAMP in comparison with whole C. atrox venom. Lanes, 1—undigested fibrinogen, 2—fibrinogen incubated with whole venom (100μg/mL), fibrinogen incubated with CAMP (100μg/mL) after 30 (3), 60 (4) and 90 (5) minutes, fibrinogen incubated with CAMP (50μg/mL) after 30 (6), 60 (7) and 90 (8) minutes, and CAMP alone (9). Representative aggregation traces (D) and data (E) demonstrate the impact of CAMP on cross-linked collagen related peptide (CRP-XL)-induced human platelet (PRP) aggregation. Data represent mean ± S.D. (n = 3). The p values shown are as calculated by One-way ANOVA followed by post hoc Tukey's test using GraphPad Prism (**p<0.01, ***p<0.001 and ****p<0.0001).