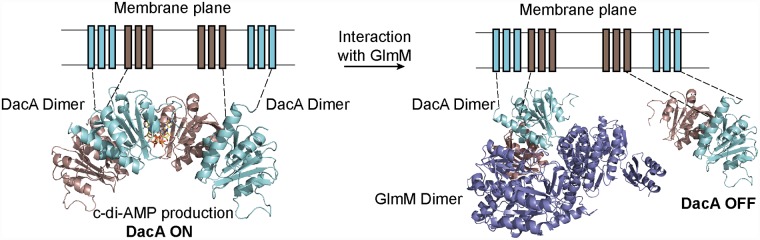
Fig 9. Proposed model of DacA inhibition by GlmM.
(Left) For active c-di-AMP synthesis, two (or more) DacA dimers interact forming catalytically active head-to-head oligomers. (Right) When GlmM binds to a DacA dimer, we speculate that this will prevent DacA from forming higher oligomers thereby turning off c-di-AMP production. GlmM dimer is colored in purple and a DacA dimer is colored in cyan (chain A) and brown (chain B). Bound ATP is colored in yellow. DacA transmembrane helices are modeled on the membrane plane and connected to the N-terminal helices of the DacACD domain by dashed lines.