Figure 4. Casein kinase II-dependent phosphorylation regulates importin α palmitoylation and spindle and nuclear size.
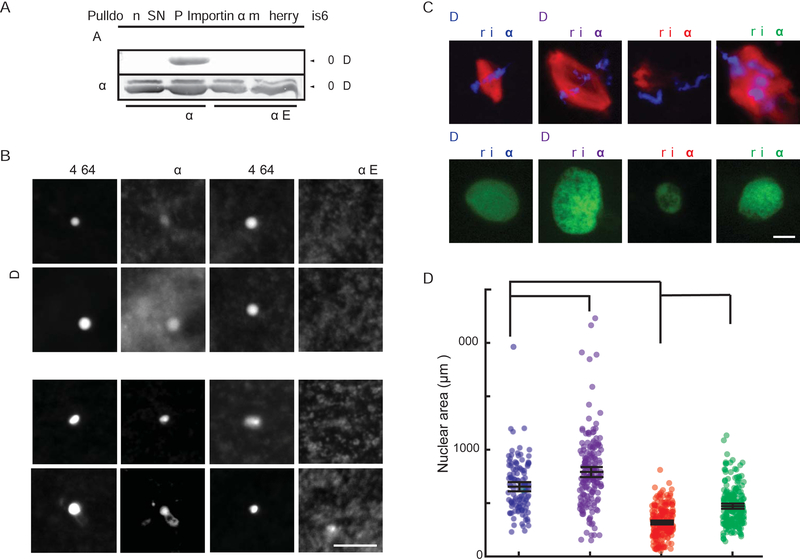
(A) Blot of immunoprecipitated recombinant mcherry-SNAP-tagged wild-type and phosphomimetic mutant importin α proteins retrieved from Xenopus egg extract probed with streptavidin following Acl-biotin exchange chemistry (Wan et al., 2007). Hydroxylamine (HA) cleaves palmitate from cysteine residues to reveal a free thiol that reacts with HPDP-biotin. HA was omitted as a negative control.
(B) Fluorescence images of GFP-tagged wild-type importin α or importin α E added to Xenopus egg extract co-stained with FM 4–64X to visualize plasma membrane lipid derived vesicles, in the presence of DMSO control or Palmostatin. Like importin α-NP, importin α-E did not localize to vesicles under any condition. Scale bar, 10 μm.
(C) Fluorescence images of metaphase spindles and interphase nuclei in egg extract reactions containing DMSO or 50 μM Quinalizarin (Qz), an inhibitor of casein kinase II (CKII). Size increases were reversed by addition of importin α-NP, although spindle assembly was aberrant.
(D) Quantification of nuclear areas in part C. Mean ± SD, 122 nuclei from 3 extracts. * = p < .05, *** = p < .0005.