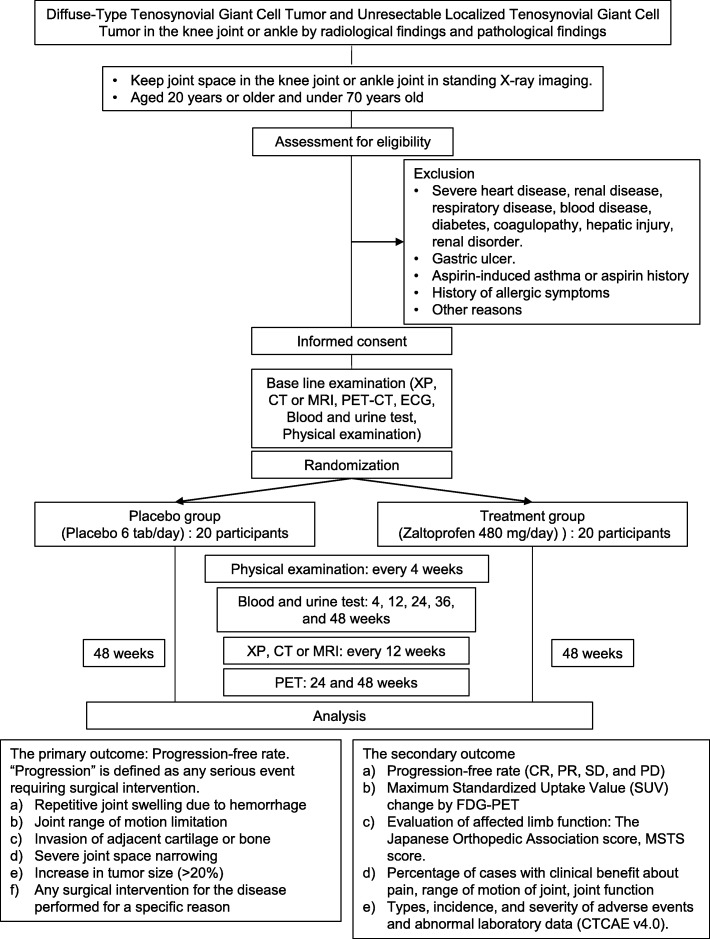
Fig. 1.
Scheme of this study protocol. CT, computed tomography; ECG, electrocardiogram; MRI, magnetic resonance imaging; PET, positron emission tomography; XP, X-ray photograph; CTCAE, common terminology criteria for adverse events; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; FDG, 18F-fluorodeoxyglucose; MSTS, Musculoskeletal Tumor Society