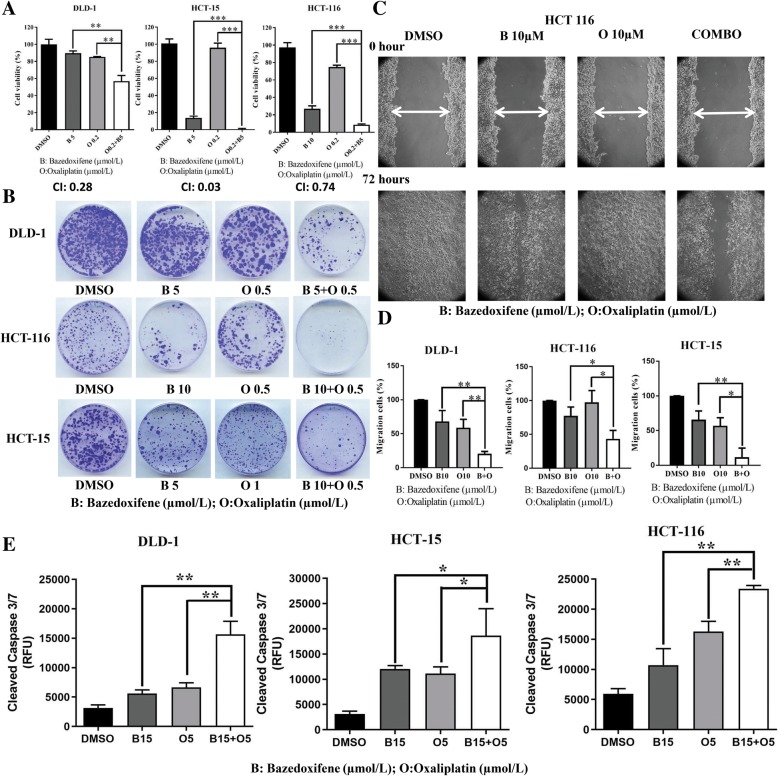
Fig. 5.
Bazedoxifene shows synergistic effects in combination with oxaliplatin. a: DLD-1, HCT-15, and HCT-116 cells were seeded in 96-well plates at a density of 3000 cells per well, incubated overnight and then treated with bazedoxifene, oxaliplatin or bazedoxifene and oxaliplatin at the indicated doses for 72 h. Cell viability was determined by MTT assay. The CI values of all combination treatments were calculated by CompuSyn software. b: DLD-1, HCT-15, and HCT-116 cells were treated with bazedoxifene, oxaliplatin or bazedoxifene and oxaliplatin combination at the indicated doses for 72 h. After treatment, the same numbers of cells were seeded and cultured in a drug-free medium for 1–2 weeks. Colonies were fixed by ice-cold methanol and stained with 1% crystal violet. c: A representative picture shows HCT-116 cells in a wound-healing assay. It was conducted by scratching the cells with a yellow pipette tip when HCT-116 cells grew into a monolayer. Then, cells were treated with 10 μM bazedoxifene, 10 μM oxaliplatin and 10 μM bazedoxifene with 10 μM oxaliplatin in combination. Cells were allowed to migrate into the scratched area for 24 h (DLD-1 and HCT-15 cells) or 72 h (HCT-116 cells). The white arrows indicate the gap in scratched area. D: The percentage of migrating area in wound-healing assay was quantified in HCT-116, DLD-1 and HCT-15 cells. E: Eight thousand cells per well of DLD-1, HCT-15, and HCT-116 cells were treated with 15 μM bazedoxifene, 5 μM oxaliplatin and a combination of 15 μM bazedoxifene with 5 μM oxaliplatin for 4 h. The level of cleaved caspase-3/7 (RFU) was measured using Caspase-3/7 Fluorescence Assay kit. (*, p < 0.05; **, p < 0.01; ***, p < 0.001)