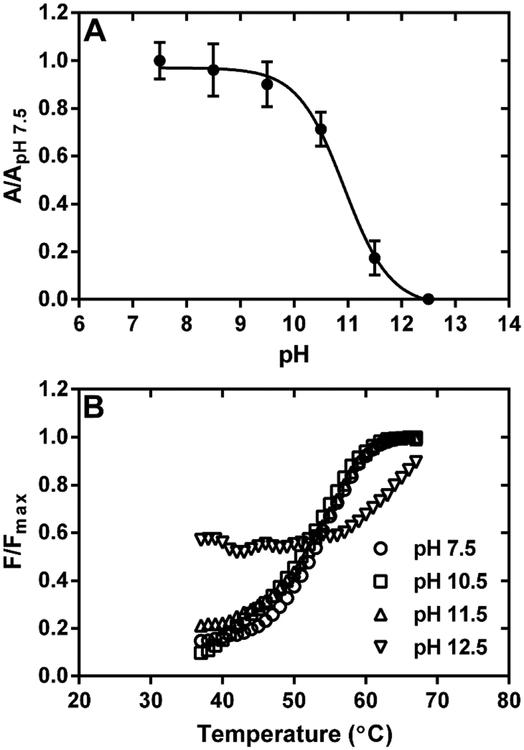
Figure 3.
Effect of solution pH on the interaction of Purβ with Acta2 ssDNA. (A) The binding of full-length Purβ (1.0 nM) to PE32-bF ssDNA (0.5 nM) was assessed in assay buffer without MgCl2 at pH ranging from 7.5 to 12.5. Solid-phase Purβ-PE32-bF complexes were detected by ELISA using rabbit anti-mouse Purβ 210–229 as the primary antibody. Nonspecific background absorbance at 405 nm in control wells without any DNA was subtracted from the signal generated in PE32-bF-coated wells. Background corrected A405 values measured at each pH were normalized by dividing by the mean A405 value determined at pH 7.5 (mean ± SD, n = 4). (B) Effect of solution pH on the thermostability of Purβ. The unfolding of full-length Purβ was evaulated by thermal shift assay at a protein concentration of 2.8 μM in 20 mM HEPES, 150 mM NaCl, 10 mM β-mercaptoethanol adjusted to pH 7.5, 10.5, 11.5, or 12.5.