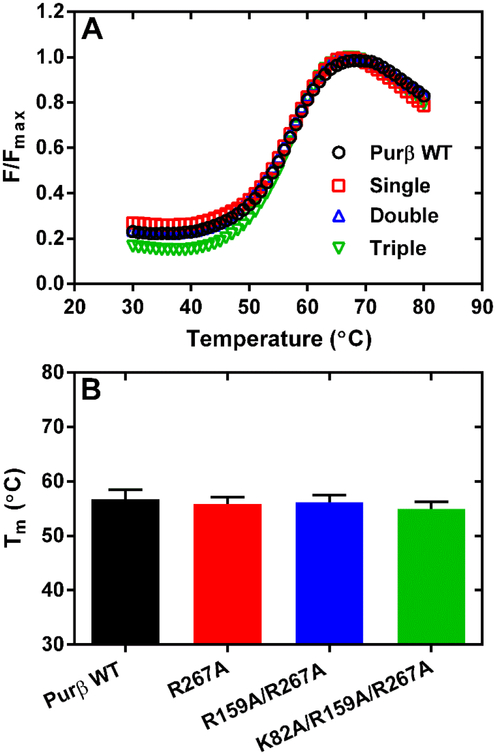
Figure 5.
Assessment of the relative thermostability of purified Purβ point mutants. (A) The unfolding of wild-type (WT) Purβ in comparison to point mutants R267A (single), R159A/R267A (double), and K82A/R159A/R267A (triple) was evaulated by thermal shift assay at a protein concentration of 2.8 μM in 50 mM sodium phosphate pH 8.0, 300 mM NaCl, 10 mM imidazole, 10 mM β-mercaptoethanol. (B) Bars show the calculated Tm of each protein determined at multiple protein concentrations ranging from 1.0 to 8.5 μM (mean ± SD, n = 6–10).