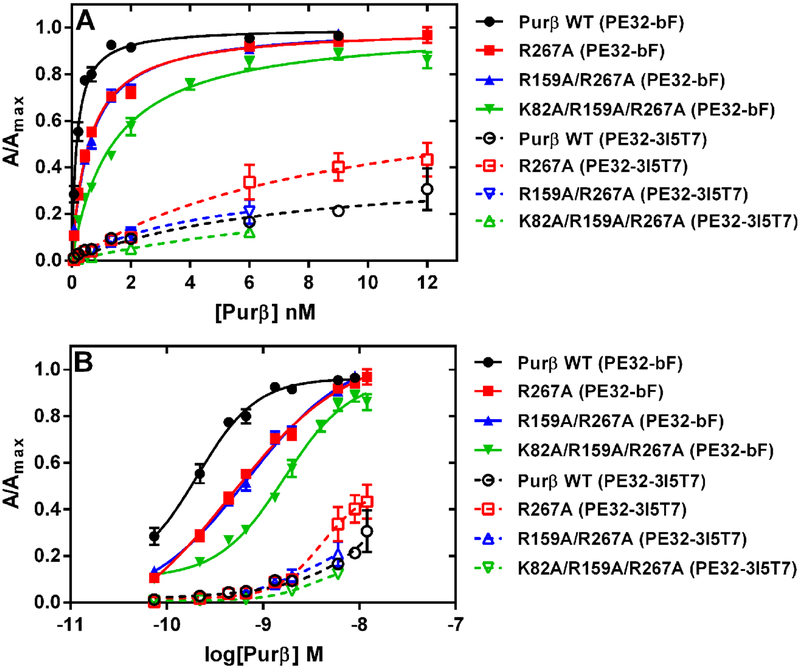
Figure 6.
Effect of R/K point mutations on the interaction of Purβ with Acta2 ssDNA. (A and B) The binding of wild-type (WT) Purβ and the indicated single (R267A), double (R159A/R267A), or triple (K82A/R159A/R267A) point mutants to 0.5 nM PE32-bF ssDNA (filled symbols) or 0.5 nM mutated PE32–3I5T7 ssDNA (open symbols) was evaluated by ELISA. Solid-phase Purβ-ssDNA complexes were detected using rabbit anti-mouse Purβ 210–229 as the primary antibody. (A) Protein concentration ranges were chosen to achieve saturable binding to the PE32-bF probe. Binding isotherms were generated by fitting data points obtained from multiple, independent titration experiments (n = 3–5) to the equation for a rectangular hyperbola. (B) Replot of the same datasets fit to a log(agonist) vs. response (four parameters) equation. The apparent Kd (A) or EC50 (B) of each Purβ protein tested for the PE32-bF probe was extrapolated accordingly.