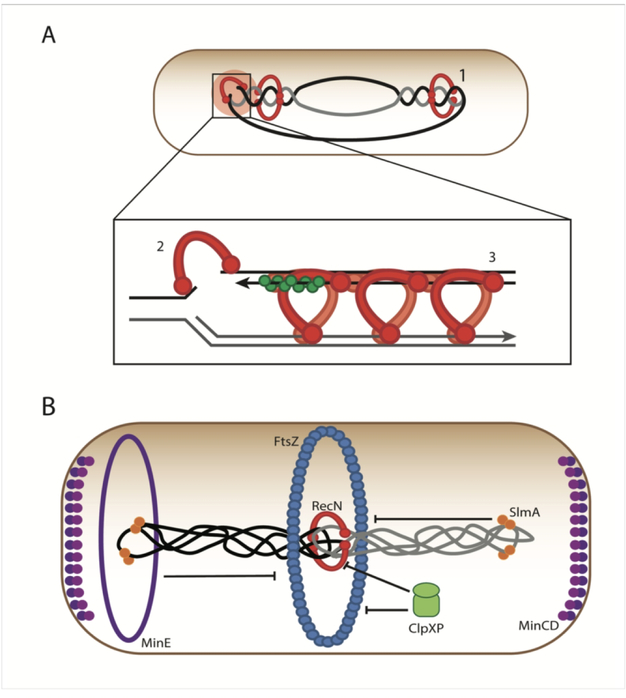
Figure 3.
Part A. RecN has been implicated in several roles in coordinating repair to DNA damage. (1) Normally, bidirectional replication leads to the formation of pre-catenanes, or inter-wound daughter strands. In the absence of DNA damage, the daughter strands are separated by Topoisomerase IV, allowing sister chromatid segregation. When DNA damage triggers the SOS Response, RecN may act to hold these precatenanes together, preventing segregation. This acts to keep the sister chromatids close, facilitating template-guided DNA damage repair (Vickridge et al., 2017). (2) RecN is also thought to perform an end-joining function during Double-Strand Break Repair (Meddows et al., 2005; Grove et al., 2009). (3) RecN may interact with RecA to facilitate homology search and strand invasion during homologous recombination of double-strand breaks (Uranga et al., 2017). Note that in all sections, whether RecN is diagrammed as part of a dimer or polymer, it is pictured as either an open circle or a helical structure as suggested by Pellegrino et al. (Pellegrino, Radzimanowski, de Sanctis, Boeri Erba, McSweeney, and Timmins, 2012b). Part B. Prior to cell division, the Z-ring forms via polymerization of FtsZ protein. This polymerization is dynamic and modulated by several proteins. It is known that the oscillating Min system prevents assembly of the Z-Ring at the poles via the action of MinC. The Nucleoid Occlusion system prevents the Z-Ring from forming prematurely over the nucleoid prior to complete of DNA replication via the protein SlmA. ClpXP has been shown to directly modulate the assembly of FtsZ polymers. During times of cell stress, the SOS response may be induced, triggering the expression of the protein RecN. RecN is known to coordinate double strand break repair. We postulate that early in an SOS Response, it acts as a cohesion-like protein to hold together the sister nucleoids. This provides an opportunity for the cell to repair DNA via homologous recombination using the newly replicated sister chromatid as a template. To ensure cell division does not progress before damage is repaired, high levels of RecN inhibit division. This provides a second way (in addition to SulA-dependent inhibition of FtsZ polymerization) for the cell to inhibit division while allocating resources to fix the damage. It is possible that RecN works with other SOS proteins in this function. This function is moderated by degradation by ClpXP. In the study above where RecN is overproduced in the absence of ClpXP, these extraordinarily high levels of RecN cause cell division defects.