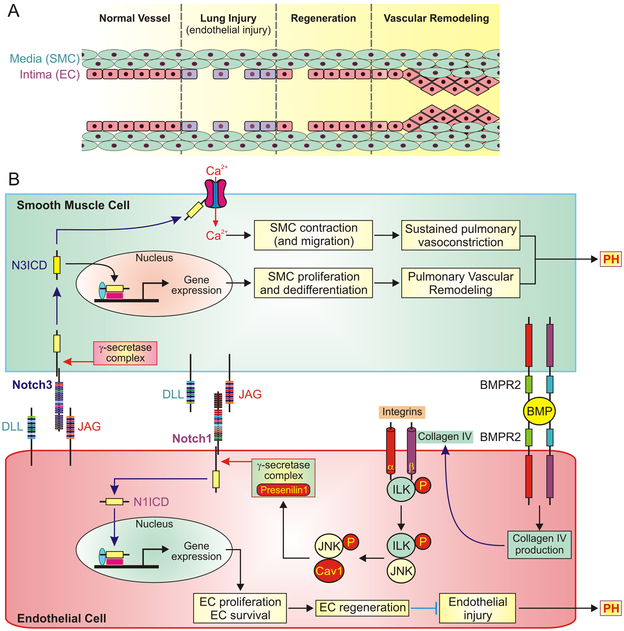
Figure 1. The proposed divergent role of Notch signaling in endothelial and smooth muscle cells.
A, The potential structural changes in endothelial monolayer of pulmonary vessel during lung vascular injury, endothelial cell (EC) regeneration and vascular remodeling. Endothelial injury results in loss of smooth muscle cell (SMC)-EC contact in the intima of injured vessels. Activated Notch pathway in EC promotes endothelial regeneration in response to lung injury but hyperactive Notch may lead to hyperproliferation of EC/SMC and concentric pulmonary artery wall thickening. B, The proposed mechanisms of Notch-mediated SMC proliferation (upper part of the panel) and EC regeneration (lower part of the panel) by EC-SMC contact and BMPR2 signaling. The mechanism involved in EC regeneration is initiated by BMPR2-dependent production of collagen IV and subsequent activation of integrin-linked kinase (ILK) and phosphorylation of the signaling molecule c-Jun N-terminal kinase (JNK) in EC. Targeting the phosphorylated JNK to caveolin 1 (Cav1) stabilizes presenilin 1 in γ-secretase complex and eventually activating Notch1. The N1ICD-associated gene expression results in EC proliferation and survival and leads to EC regeneration and endothelial repair. Re-established EC-SMC contact also activates Notch3 in SMC, especially in SMC with upregulated Notch3, and release N3ICD into the cytoplasm. N3ICD can go through the canonical pathway to stimulate gene expression involved in SMC proliferation and dedifferentiation and eventually results in pulmonary vascular remodeling. N3ICD may also functionally interact with Ca2+ channels in the plasma membrane, increases Ca2+ influx and subsequently causes SMC contraction and pulmonary vasoconstriction.