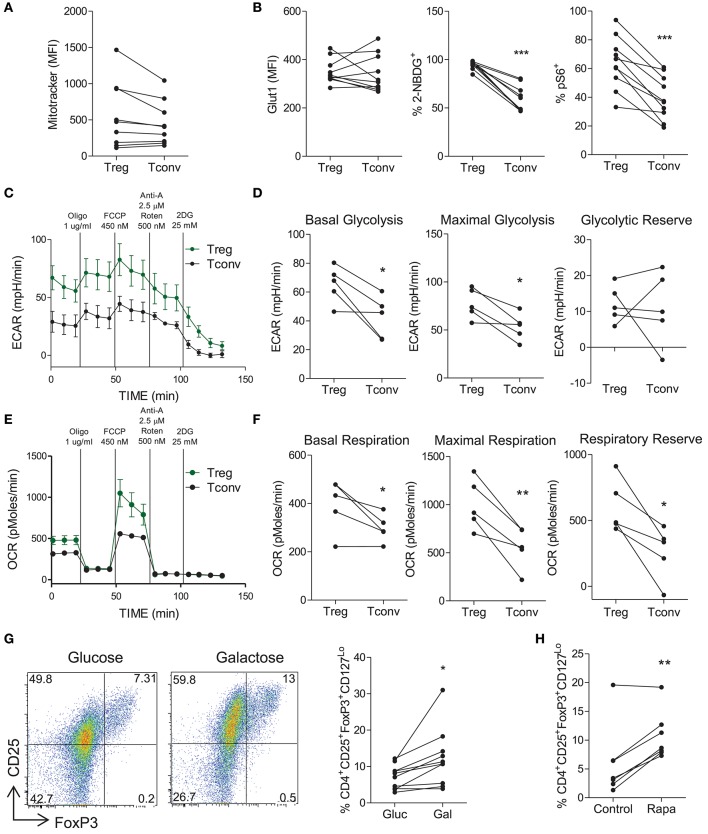
Figure 4.
Treg cells demonstrate increased glycolysis and oxidative phosphorylation relative to conventional T cells, but do not depend on glycolysis. PBMC were isolated from healthy controls and cells were stained ex vivo for CD4, CD25, CD45RO, CD127 and MitoTracker® Green. MitoTracker® Green staining in CD4+CD45RO+CD25+CD127Lo (Treg) and CD4+CD45RO+NotCD25+CD127Lo (Tconv) (n = 9) (A). Memory CD4+ T cells were isolated from PBMC by magnetic separation and stimulated with anti-CD3 and irrAPC. Cells were stained for CD4, CD25, CD127, FoxP3, Glut1, pS6, and 2-NBDG uptake was measured. The expression of Glut1 in Treg and Tconv at 24 h stimulation, 2-NBDG at 72 h, and pS6 at 24 h (n = 10) (B). Treg and Tconv cells were cell sorted from PBMC. Cells were cultured for 6 d in the presence of anti-CD3, irrAPC and IL-2. Cells were stimulated for 18 h with PMA and ionomycin prior to Seahorse extracellular flux analysis. Representative plot of ECAR over time for Treg and Tconv (C). Basal glycolysis, maximal glycolytic capacity and glycolytic reserve rates for Treg and Tconv (n = 5) (D). Representative plot of OCR over time for Treg and Tconv (E). Basal respiration, maximal respiratory capacity and respiratory reserve rates for Treg and Tconv (n = 5) (F). Memory CD4+ T cells were isolated by magnetic separation and cultured for 5 d in the presence of anti-CD3 and irrAPC in glucose-free medium supplemented with glucose (Gluc) or galactose (Gal). Cells were stained for CD4, CD25, CD127, and FoxP3; and analyzed by flow cytometry. Representative dot plots accompanied by the frequencies of Treg cells in glucose or galactose medium (n = 9) (G). Memory CD4+ T cells were cultured for 5 d with anti-CD3 and irrAPC in the presence or absence of rapamycin (Rapa). Cells were stained for CD4, CD25, CD127, and FoxP3; and analyzed by flow cytometry. The frequencies of Treg cells following control or Rapa treatment (n = 6) (H). *p < 0.05, **p < 0.01, ***p < 0.001.