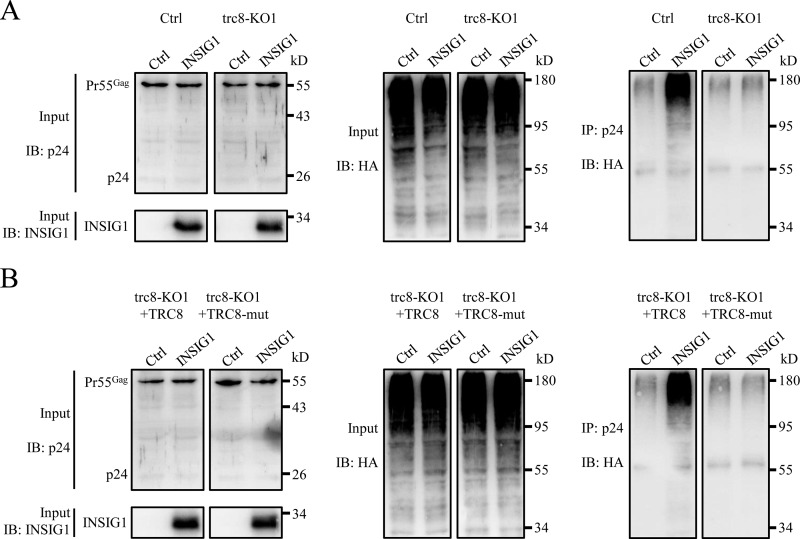
Figure 8.
INSIG1 increased the ubiquitination of Gag via TRC8. A, the stable trc8 knockout cell line (trc8-KO1) and control 293T cells were transfected with plasmids of NLENY1-ES-IRES, HA-ubiquitin, together with or without INSIG1. The cells were then treated with chloroquine at 6 h post-transfection and cultured for another 24 h prior to harvesting. Cell lysates were immunoprecipitated with anti-p24 antibody, and corresponding proteins were analyzed by Western blotting. As shown in the right panel, in control cells, but not in trc8 knockout cells, Gag could be more efficiently ubiquitinated in the presence of INSIG1. B, WT TRC8 or its enzymatic defective mutant (TRC8-mut) were supplemented into the experimental system used in A in trc8 knockout cells. Cell lysates were immunoprecipitated with anti-p24 antibody, and corresponding proteins were analyzed by Western blotting. Reintroduction of TRC8, but not its mutant, restored the function of INSIG1 on Gag ubiquitination.