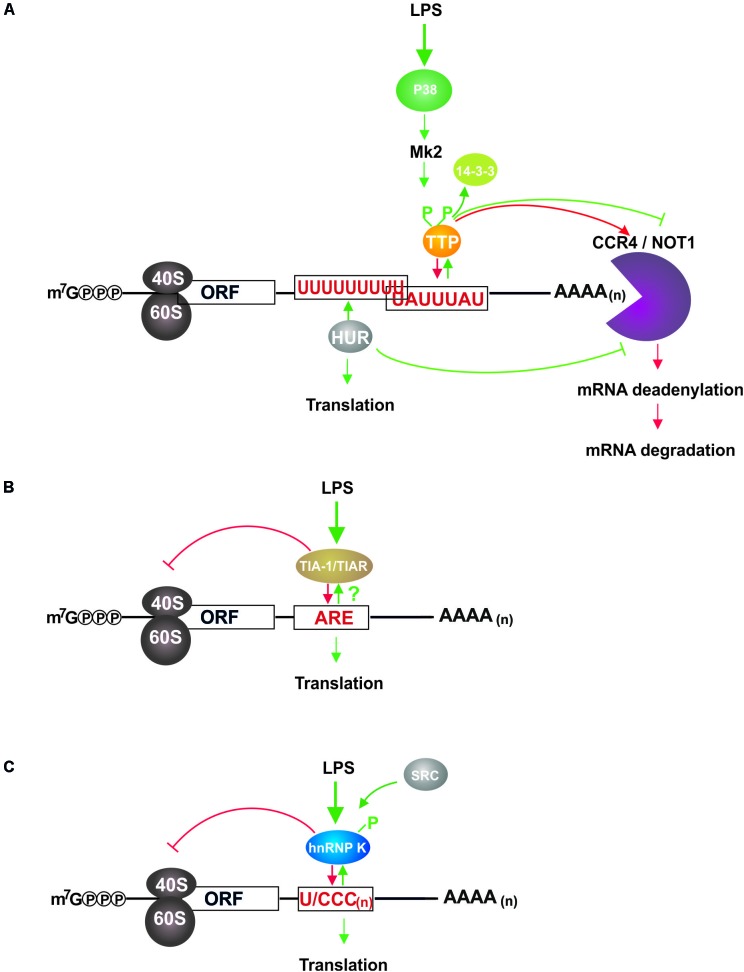
FIGURE 1.
LPS induced impact of TTP, HUR, TIAR, and hnRNP K on mRNAs in macrophages. (A) TTP and HUR interact with U-rich elements in the 3′UTR of target mRNAs and regulate their stability and translation (Stoecklin et al., 2008; Tiedje et al., 2012, 2016; Sedlyarov et al., 2016). In non-induced cells bound TTP recruits the CCR4/NOT1 complex, which initiates 3′UTR deadenylation and 3′ to 5′ mRNA decay. LPS induced TLR4 signaling activates MK2, which phosphorylates TTP leading to its sequestration by 14-3-3 proteins and subsequent abolition of deadenylation complex association. Following T118 phosphorylation by MK2 HUR accumulates in the cytoplasm where it mediates mRNA stabilization, disables decay complex formation and enhances translation. By controlling mRNA decay, TTP curtails the synthesis of inflammation related proteins in non-induced macrophages, and regulates their balanced expression in response to LPS conjointly with HUR. (B) TIAR binding to AREs causes translational repression of target mRNAs, which is diminished by a so far unknown mechanism in LPS activated macrophages, resulting in elevated synthesis of cytokines (Piecyk et al., 2000; Kharraz et al., 2016). TIAR dampens the expression of immune response associated proteins, which can be activated immediately in response to LPS. (C) HnRNP K bound to U/CCC(n) elements in the mRNA 3′UTR inhibits target mRNA translation, but is released from the binding site following c-Src catalyzed tyrosine phosphorylation that is initiated in response to LPS dependent macrophage activation (Liepelt et al., 2014). Thereby a rapid LPS response facilitated by straight signaling molecule synthesis can be established.