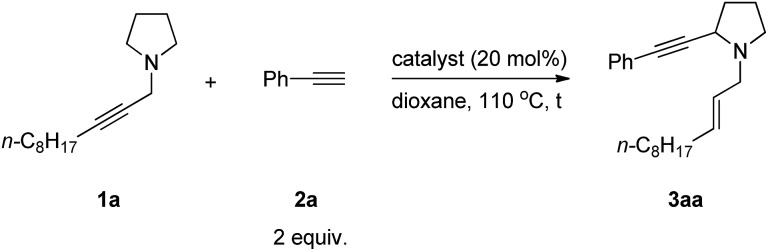
Table 1. Optimization of catalytic α-alkynylation of 1-(2-alkynyl) cyclic amine 1a with 2a a .
| ||||
| Entry | Catalyst | Time (h) | Yield of 3aa b (%) | Recovery of 1a b (%) |
| 1 | CuBr | 12 | 13 | 64 |
| 2 | CuBr2 | 10 | 13 | — |
| 3 | ZnCl2 | 10 | 24 | — |
| 4 | ZnBr2 | 10 | 39 | — |
| 5 | AgOTf | 12 | 5 | 95 |
| 6 | CdI2 | 10 | 40 | — |
| 7 | CdBr2 | 24 | 42 | 53 |
| 8 c | CdBr2 | 36 | 56 | 20 |
| 9 d | CdBr2 | 12 | 47 | 26 |
aThe reaction was conducted using 1a (1.0 mmol) and alkyne 2a (2.0 mmol) in 6 mL of dioxane at 110 °C.
bDetermined by 1H NMR analysis with CH2Br2 as the internal standard.
cThe reaction was conducted at 120 °C.
dThe reaction was conducted at 130 °C.
