Table 2. Optimization of reaction conditions for catalytic α-alkynylation of N-internal 2-alkynylic cyclic amine 1a with 2a a .
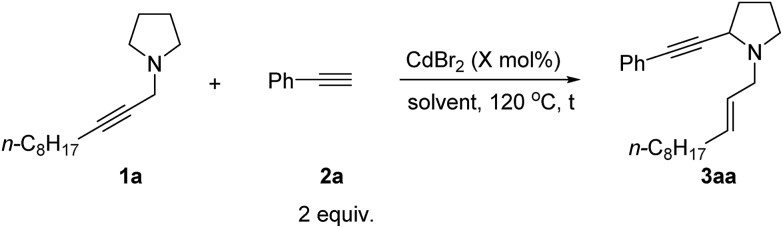
| |||||
| Entry | X | Solvent | t (h) | Yield of 3aa b (%) | Recovery of 1b b (%) |
| 1 | 20 | DMF | 23 | 43 | 25 |
| 2 | 20 | DMSO | 23 | 20 | 35 |
| 3 | 20 | Toluene | 23 | 40 | — |
| 4 | 20 | THF | 23 | 48 | — |
| 5 | 20 | DCE | 23 | 3 | — |
| 6 | 20 | CH3CN | 23 | 39 | — |
| 7 | 20 | t BuOMe | 36 | 63 | — |
| 8 | 15 | t BuOMe | 36 | 64 | — |
| 9 c | 10 | t BuOMe | 36 | 66 | — |
| 10 | 5 | t BuOMe | 36 | 69 | 10 |
aThe reaction was conducted using 1a (0.5 mmol) and alkyne 2a (1.0 mmol) in 3 mL of solvent.
bDetermined by 1H NMR analysis with CH2Br2 as the internal standard.
cThe reaction was conducted using 1a (1.0 mmol) and alkyne 2a (2.0 mmol) in 6 mL of tBuOMe at 120 °C.
