Table 3. The scope of catalytic α-alkynylation of N-internal 2-alkynylic cyclic amines a .
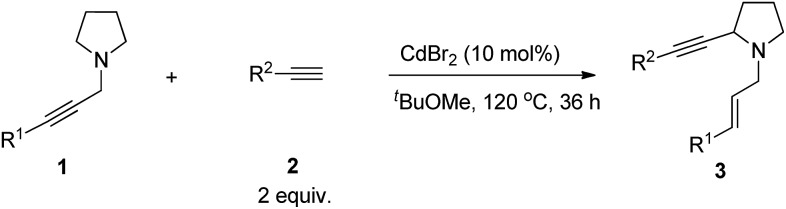
| |||
| Entry | 1 (R1) | 2 (R2) | Isolated yield of 3 b (%) |
| 1 | n-C8H17 (1a) | C6H5 (2a) | 63 (3aa) |
| 2 | n-C8H17 (1a) | p-MeC6H4 (2b) | 51 (3ab) |
| 3 c | n-C8H17 (1a) | p-MeOC6H4 (2c) | 45 (3ac) |
| 4 | n-C8H17 (1a) | p-FC6H4 (2d) | 55 (3ad) |
| 5 | n-C8H17 (1a) | p-ClC6H4 (2e) | 67 (3ae) |
| 6 | n-C8H17 (1a) | m-ClC6H4 (2f) | 66 (3af) |
| 7 | n-C8H17 (1a) | p-O2NC6H4 (2g) | 60 (3ag) |
| 8 | n-C8H17 (1a) | p-EtOOCC6H4 (2h) | 60 (3ah) |
| 9 | n-C8H17 (1a) | p-NCC6H4 (2i) | 61 (3ai) |
| 10 | n-C8H17 (1a) | p-AcC6H4 (2j) | 59 (3aj) |
| 11 d | n-C8H17 (1a) | n-C8H17 (2k) | 31 (3ak) |
| 12 e | n-C8H17 (1a) | Cy (2l) | 40 (3al) |
| 13 f | n-C8H17 (1a) | TMS (2m) | 76 (3am) |
| 14 | Cy (1b) | C6H5 (2a) | 65 (3ba) |
| 15 g | n-C4H9 (1c) | C6H5 (2a) | 57 (3ca) |
| 16 h | (CH3)2(OH)C (1d) | C6H5 (2a) | 62 (3da) |
| 17 i | H (1e) | C6H5 (2a) | 47 (3ea) |
| 18 i | H (1e) | p-MeOC6H4 (2c) | 45 (3ec) |
| 19 i | H (1e) | p-FC6H4 (2d) | 48 (3ed) |
| 20 i | H (1e) | p-ClC6H4 (2e) | 46 (3ee) |
| 21 j | Ph (1g) | C6H5 (2a) | 40 (3ga) |
aThe reaction was conducted using 1 (1.0 mmol) and 1-alkyne 2 (2.0 mmol) in 6 mL of MTBE at 120 °C for 36 h.
b E/Z > 20 : 1, if any.
c22% of 1a was recovered.
d20% of CdBr2 was used and 27% of 1a was recovered.
e50% of 1a was recovered.
fThe reaction was conducted at 130 °C and 3% of 1a was recovered.
g15% of CdBr2 was used.
hThe reaction was conducted at 130 °C and 4% of 1d was recovered.
iThe reaction was conducted using 1e (1.0 mmol), alkyne 2 (2.0 mmol) and ZnI2 (0.3 mmol) in 6 mL of dioxane at 110 °C for 10 h.
jThe reaction was conducted in 6 mL of toluene and 25% of 1g was recovered.
