Figure 2.
De Novo Variants in MAPK8IP3 and Structural Effects in the Leucine Zipper Domain
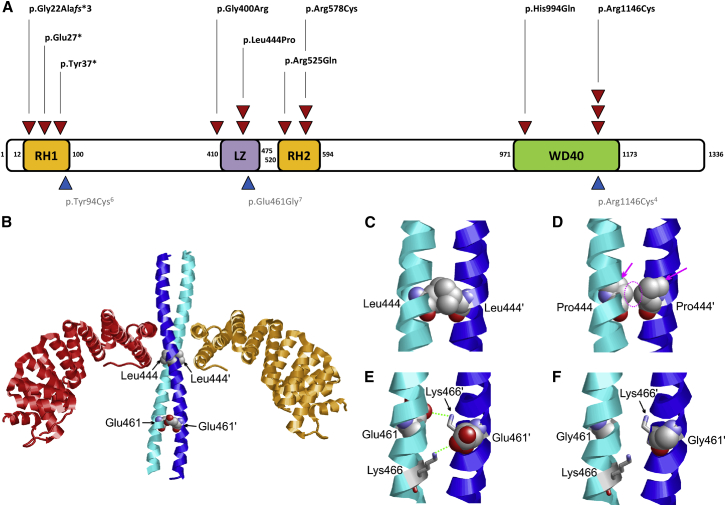
(A) Location of de novo single-nucleotide variants in MAPK8IP3 with respect to domain structure (GenBank: NM_015133.4). Red triangles = individuals from this cohort. Blue triangles = published variants in individuals with a neurodevelopmental disorder. Abbreviations are as follows: LZ =leucine zipper; RH1 and RH2 = Rab-interacting lysosomal protein (RILP) homology 1 and 2.
(B) Crystal structure of the MAPK8IP3 leucine zipper (cyan and blue) in complex with two TPR domains of the kinesin light chain (orange and red). The sites of the sequence variants detected in the present study are shown in space-filled presentation.
(C) The Leu444 sidechains of the homodimeric leucine zipper interact with each other (a prime denotes residues of the second subunit).
(D) In the p.Leu444Pro variant, these interactions are lost as a result of the shorter proline sidechain (dotted circle). In addition, the cyclic proline sidechain causes a steric clash with the backbone of adjacent helix residues (magenta arrows). Both effects are expected to cause a drastic destabilization of the helix and to impede homodimer formation.
(E) The Glu461 side chain forms weak inter-subunit electrostatic interactions with Lys466 (green dotted lines).
(F) The p.Glu461Gly variant cannot form these polar interactions because there is no charged sidechain.