Summary
We have reported that 3a,4,5,9b-tetrahydro-4-(1-naphthalenyl)-3H-cyclopentan[c]quinoline-8-sulfonamide (TQS), α7 nicotinic acetylcholine receptor (nAChR) positive allosteric modulator (PAM) reduces lipopolysaccharide (LPS)-induced hyperalgesia and allodynia in mice. The objective of the present study was to determine the effects of TQS on LPS-induced activation of hippocampal inhibitor of κB (IκB) and cluster of differentiation 11b (CD11b) gene expression involving hyperalgesia and allodynia in mice. We also examined the effects of TQS on microglial phenotype following LPS administration. Pretreatment of TQS (4 mg/kg) reduced the expressions of IκB and CD11b mRNA. Pretreatment of methyllycaconitine (3 mg/kg), an α7 nAChR antagonist, reversed TQS-induced decrease in IκB and CD11b mRNA expressions in the hippocampus indicating the involvement of α7 nAChR. In addition, TQS (4 mg/kg) reversed the LPS-induced microglial morphological changes. These results suggest that TQS reduces LPS-induced IκB and CD11b gene expression and microglial activation associated with hyperalgesia and allodynia by targeting microglial α7 nAChR in the hippocampus.
Keywords: Nicotinic receptor, α7 positive allosteric modulator, microglia, hippocampus, pain, mice
1. Introduction
The α7 nicotinic acetylcholine receptors (nAChRs) are composed of α subunits that assemble to form homo-pentomers. These receptors have been identified to be highly expressed on microglia within hippocampus (1,2). Moreover, brain nicotinic cholinergic pathway has been proposed to regulate microglial activation involving α7 nAChRs (3). The α7 agonists have been shown to reduce hyperalgesia and allodynia in a number of animal models (4–6). However, these receptors undergo rapid desensitization upon agonist binding (7), and adopt stable non-conducting (desensitized) conformations (8). One alternative approach to target α7 nAChRs, is the use of type II positive allosteric modulators (PAMs) that can efficaciously prevent normal desensitization and can even reactivate desensitized α7 nAChR (8,9). The α7 nAChR type II PAMs were found to decrease pain sensitivity in animal models in the spinal cord (10,11). However, the antinociceptive effects of α7 nAChR type II PAMs on pain sensitivity in the hippocampus remain unknown.
Emerging evidence indicates that hippocampus plays a critical role in pain perception and processing (12,13) and this limbic structure is densely populated with microglia cells compared to other brain regions (14). This suggests a potential role of hippocampal microglia during hyperalgesia and allodynia. Furthermore, recent studies indicate that microglial cells are important regulators in the development and maintenance of pain-like symptoms (15). Furthermore, ramified “resting state” microglial cells do not exhibit activation for inflammatory signaling pathway reflecting in binding of inhibitor of κB (IκB) to nuclear factor-κB (NF-κB) within cytoplasm (16,17). However, IκB becomes phosphorylated and exposed to proteolytic degradation upon an appropriate stimulation (18). The degradation of IκB protein unmasks NF-κB from inactive to active state and the active NF-κB is then translocated to nucleus and positively regulates the transcription of various pain mediating genes, including IκB mRNA (19,20). A growing body of evidence has shown the involvement of increased IκB mRNA expression in the mediation of pain-like symptoms (6,21). Therefore, the evidence indicates that increased IκB mRNA and cluster of differentiation 11b (CD11b) mRNA, a microglial activation marker in the brain, expressions occur simultaneously during hyperalgesia and allodynia (6,15,22).
Recently, we have shown that the α7 PAM 3a,4,5,9btetrahydro-4-(1-naphthalenyl)-3H-cyclopentan[c] quinoline-8-sulfonamide (TQS) (9) reduced lipopolysaccharide (LPS)-induced hyperalgesia and allodynia due to decreased microglial activation in the hippocampus involving α7 nAChRs (23). However, the effects of TQS on IκB remain unknown during LPS-induced hyperalgesia and allodynia in the hippocampus. In the present study, we have examined the effects of TQS on IκB mRNA and CD11b mRNA expressions and microglial phenotype in the hippocampus following LPS administration in mice. Additionally, we have determined the effects of methyllycaconitine (MLA), an α7 nAChR antagonist, on IκB mRNA and CD11b mRNA expressions in the hippocampus.
2. Materials and Methods
2.1. Animals
Male C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). The animals were housed four per cage (29 × 18 × 12 cm), under standard laboratory conditions (22 ± 2°C, relative humidity 50–60%) with a 12-h light/dark cycle (lights on from 06:00 AM to 6:00 PM) having unlimited access to food and water. Mice were 10–12 weeks of age at the beginning of the experiment. All procedures were in compliance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at South Dakota State University. Good Laboratory Practice and ARRIVE guidelines were obeyed. All efforts were attempted to ensure limited animals suffering.
2.2. Drugs
The LPS (055:B5) and MLA were purchased from Sigma-Aldrich (St. Louis, MO, USA). The TQS was purchased from Tocris Bioscience (Ellisville, MO, USA) and was dissolved in normal saline (0.9% NaCl) containing 0.5% tween 80 and 1% dimethyl sulfoxide (DMSO). LPS and MLA were dissolved in normal saline. All drugs were administered intraperitoneally in a volume of 10 mL/kg of body weight.
2.3. Experimental timeline
LPS was injected as described previously (23) and TQS was administered 0.5 h before LPS administration. The MLA was given 10 min before TQS injection. Brain tissue was collected for quantitative real-time polymerase chain reaction and immunohistochemistry six h after LPS administration.
2.4. RNA isolation and cDNA synthesis
Total RNA was isolated and cDNA was synthesized as described previously with minor modifications (6). Briefly, mice were sacrificed six h after LPS administration and their hippocampi and brain stems were dissected out, frozen on dry ice, and stored in – 80°C refrigerator until further analysis. RNA was extracted from the tissue by using chloroform and isopropanol after homogenizing the tissue in the presence of Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s instructions. RNA samples were stored at – 80°C until further analysis. RNA was then reverse transcribed into first-strand cDNA using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA) in Master Cycler Personal (Eppendorf, Hauppauge, NY, USA). The reaction mixture consisted of RT random hexamer primer, dNTP Mix, multiscribe reverse transcriptase, RNase inhibitor, RT buffer, and nuclease free water to a total volume of 20 μL. The mixture was then incubated at 25°C for 10 min, at 37°C for 2 h, and at 85°C for 5 min for deactivating the enzyme. All cDNA was stored at – 80°C until quantitative real-time PCR was performed.
2.5. Quantitative real-time polymerase chain reaction
Quantitative real-time PCR was performed as described previously with minor modifications (6). Briefly, cDNA template was used for PCR reaction. Primer sequences for IκB, CD11b, and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) were obtained from Integrated DNA Technologies (Coralville, Iowa, USA). Amplification of cDNA was performed using SYBR Select PCR Master Mix Kit (Applied Biosystems by life technologies, Austin, TX, USA) in StepOnePlus quantitative real-time PCR system (Applied Biosystems, Carlsbad, CA, USA). The reaction mixture (20 μL) was composed of SYBR Select PCR Master Mix, 200 nM of prime primer, nuclease free water, and cDNA template. GAPDH primer was used as housekeeping gene. Reactions were carried out using 96 wells qPCR plate (Life Technologies, Grand Island, NY, USA). Cycling parameters were as followed; 50°C for 2 min once; 95°C for 2 min once; then 95°C for 15 s (denaturation), 60°C for 30 s (annealing), and 72°C for 1 min and 30 s (extension) for 40 cycles ending with a melting curve analysis to control amplification. The cycle threshold (Ct) obtained form PCR was used for analysis. The Ct value was determined for each gene and relative expression of each gene was calculated using delta-delta Ct method. The level of the target mRNA was quantified relative to GAPDH and presented as percentage of vehicle control.
2.6. Immunohistochemistry
Immunohistochemistry was performed as described previously with some modification (17). After sacrificing mice six h after LPS administration, their brains were harvested and fixed with 4% paraformaldehyde (Acros organics, New Jersey, USA) for 24 h. Mice brains were then cryoprotedcted at 4°C in 30% sucrose (Sigma-Aldrich, St. Louis, MO, USA) until brains sank. The 40 μm coronal sections were cut using Leica cryostat. Free floating sections were then placed in water bath maintained at 90°C having boiling 0.01 M citrate buffer (pH 6.0) for 10 min, followed by quenching in 0.3% hydrogen peroxide (Acros Organics, New Jersey, USA) in methanol for 5–10 min. Hippocampal tissue sections were then blocked in 1.5% normal goat serum (Santa Crus Biotech, Dallas, Texas, USA) at room temperature for 1 h. The sections were then incubated with primary antibody (goat anti-rabbit-Iba-1, 2.25:1000, Wako, Osaka, Japan) at room temperature for 1.5 h. The sections were then incubated in secondary antibody (biotinylated goat anti-rabbit; 5:1000, Santa Crus Biotech, Dallas, Texas, USA) at room temperature for 30 min followed by staining with avidin-biotin complex, diaminobenzidine, and mounted on superfrost plus microscope slides (Fisher Scientific, USA). Images were taken three to four random fields in the hippocampus at 20× after overnight drying with bright field microscopy. Quantification of % area occupied by microglial cells was carried out using Image J software.
2.7. Data analysis
Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons or Student’s t-test for MLA data using GraphPad Prism 5.0 (GraphPad Inc., San Diego, CA, USA). The difference between treatments was considered significant at p < 0.05. Results are expressed as mean ± S.E.M.
3. Results
3.1. Effects of TQS on LPS-induced activation of IκB mRNA expression in the hippocampus
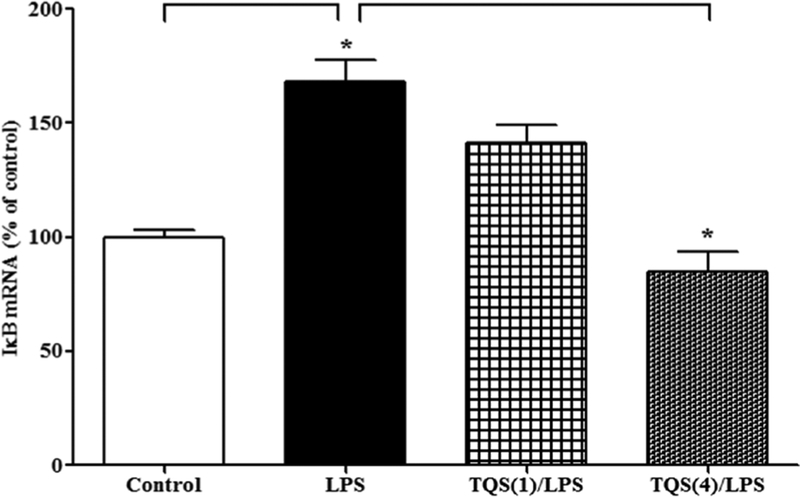
One-way ANOVA revealed that TQS significantly decreased the expression of IκB mRNA following LPS administration (Figure 1, F3,20 = 23.93; p < 0.0001). Multiple comparisons of means indicated that LPS (1 mg/kg) significantly (p < 0.001) increased the expression of IκB mRNA. Furthermore, TQS (4 mg/kg) significantly (p < 0.001) decreased IκB mRNA expression during LPS-induced hyperalgesia and allodynia in the hippocampus. Moreover, LPS did not significantly alter the expression of IκB mRNA in brain stem (control: 100 ± 7.0 vs. LPS: 115 ± 2.7).
Figure 1. Effects of TQS on LPS-induced activation of IκB mRNA expression in the hippocampus in mice.
Mice (n = 6) were administered TQS (1 or 4 mg/kg, i.p.) 0.5 h before LPS administration. Control animals received equal volume of vehicle. Data are expressed as mean ± S.E.M. * p < 0.001.
3.2. Effects of MLA on TQS-induced decrease in IκB mRNA expression in the hippocampus
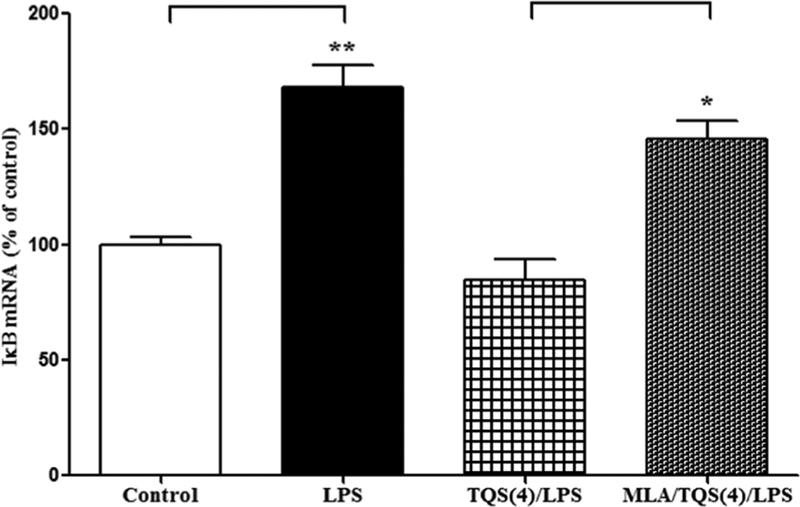
To examine the involvement of α7 nAChR, MLA, an α7 nAChR antagonist, was administered along with TQS. Student’s t-test indicated that MLA significantly (p < 0.01) reversed decrease in IκB mRNA expression (Figure 2).
Figure 2. Effects of MLA on TQS-induced decrease in IκB mRNA expression in the hippocampus.
Mice (n = 4–6) were administered TQS (4 mg/kg, i.p.) 0.5 h before LPS administration. MLA (3 mg/kg, i.p.) was given 10 min before TQS injection. Control animals received equal volume of vehicle. Data are expressed as mean ± S.E.M. *p < 0.01; **p < 0.001.
3.3. Effects of TQS on LPS-induced activation of CD11b mRNA expression in the hippocampus
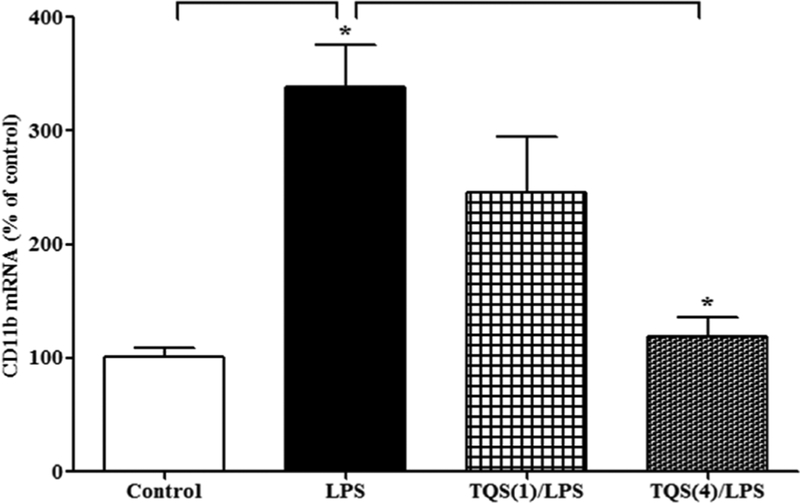
One-way ANOVA indicated that TQS significantly reduced the expression of CD11b mRNA following LPS administration (Figure 3; F3,14 = 10.36; p < 0.001). Multiple comparisons of means revealed that LPS significantly (p < 0.01) increased CD11b mRNA expression. Furthermore, TQS (4 mg/kg) significantly (p < 0.01) reduced CD11b mRNA expression in the hippocampus. Moreover, LPS did not alter the expression of CD11b mRNA in brain stem (control: 100 ± 9.4 vs. LPS: 104 ± 14.9).
Figure 3. Effects of TQS on LPS-induced activation of CD11b mRNA expression in the hippocampus in mice.
Mice (n = 4–5) were administered TQS (1 or 4 mg/kg, i.p.) 0.5 h before LPS administration. Control animals received equal volume of vehicle. Data are expressed as mean ± S.E.M. *p < 0.01.
3.4. Effects of MLA on TQS-induced decrease in CD11b mRNA expression in the hippocampus
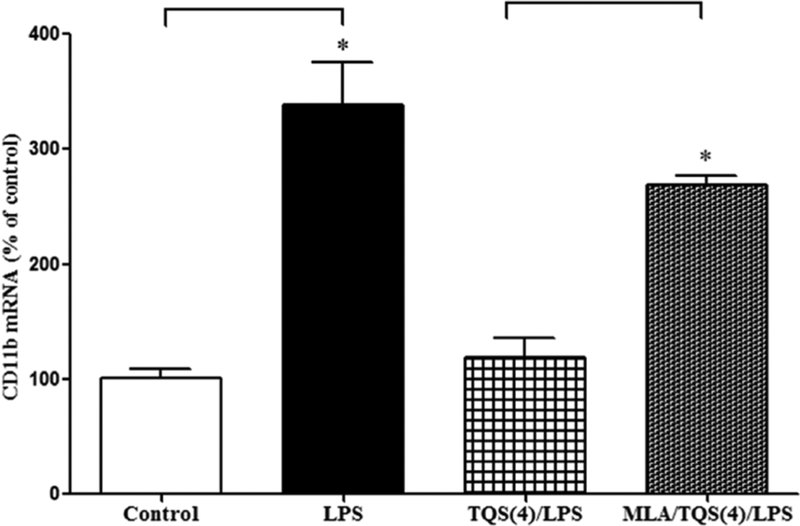
To characterize the involvement of α7 nAChR during microglial activation, MLA was administered along with TQS. Student’s t-test indicated that MLA significantly (p < 0.001) reversed reduction in CD11b mRNA expression (Figure 4).
Figure 4. Effects of MLA on TQS-induced decrease in CD11b mRNA expression in the hippocampus in mice.
Mice (n = 4–5) were administered TQS (4 mg/kg, i.p.) 0.5 h before LPS administration. MLA (3 mg/kg, i.p.) was given 10 min before TQS injection. Control animals received equal volume of vehicle. Data are expressed as mean ± S.E.M. *p < 0.001.
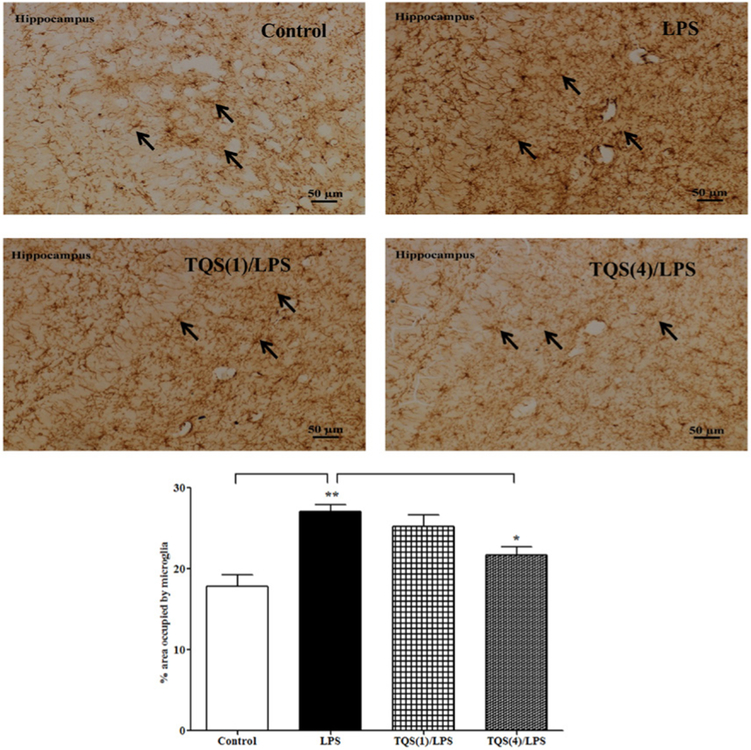
3.5. Effects of TQS on microglial cells morphology following LPS administration in the hippocampus
One-way ANOVA revealed that TQS pretreatment significantly affected microglial activation in the hippocampus (Figure 5; F3,19 = 11.83; p < 0.001). Multiple comparisons of means revealed that LPS (1 mg/kg) significantly (p < 0.001) increased microglial activation by increasing microglial morphology in the hippocampus as compared to control. Furthermore, TQS (4 mg/kg) significantly (p < 0.05) decreased microglial activation due to reduced LPS-induced morphological changes in the hippocampus compared to LPS treated group.
Figure 5. Effects of TQS on microglial cells morphology following LPS administration in the hippocampus in mice.
Representative immunoreactivity for microglial morphology in hippocampus (top panel). Mice (n = 5–6) received TQS (1 or 4 mg/kg, i.p.) 0.5 h before LPS administration. Control animals received equal volume of vehicle. Data are expressed as mean ± SEM. *p < 0.05; **p < 0.01.
4. Discussion
In the present study, we have demonstrated that pretreatment of TQS reduced the expression of LPS-induced IκB mRNA, CD11b mRNA and regulated microglial morphological changes in the hippocampus. Furthermore, we have found that pretreatment of methyllycaconitine, an α7 nAChR antagonist, reversed TQS-induced decrease in IκB mRNA and CD11b mRNA expressions in the hippocampus indicating the involvement of α7 nAChR during LPS-induced hyperalgesia and allodynia in mice. The data are consistent with microglial mechanisms involving LPS-induced hyperalgesia and allodynia (23).
Elevated level of IκB mRNA, associated with increased NF-κB activity in parallel and used as an index for increased NF-κB activation, in the CNS is associated with occurrence of pain-like symptoms (6). Moreover, the activation of NF-κB associated with pain hypersensitivity has been identified in other pain models (24,25) and is likely involved in pain hypersensitivity. Given the evidence, we propose a link between increased IκB mRNA expression and NF-κB activation involving LPS-induced hyperalgesia and allodynia in mice as previously reported (23). The pretreatment of MLA, an α7 nAChR antagonist, reversed the TQS-induced decrease in I-κB mRNA expression in the hippocampus supporting the involvement of α7 nAChRs during NF-κB inactivation. To the best of our knowledge, this is the first report which indicates that the effects of α7 nAChR PAM on the hippocampal microglia results in microglial deactivation by reducing NF-κB activation associated with LPS-induced hyperalgesia and allodynia.
It is noteworthy to mention that increased level of IκB mRNA expression is associated with NF-κB activation in the hippocampus. This activation is likely regulated by increased microglial activation as evidenced by elevated expression of CD11b mRNA expression during LPS-induced hyperalgesia and allodynia. Therefore, it is conceivable that the effects of α7 nAChR PAM in the hippocampus are mediated through microglia as LPS-induced increased expression of CD11b mRNA was significantly attenuated by TQS. On the other hand, pretreatment of MLA reversed the TQS-induced decrease in CD11b mRNA expression suggesting an activation of α7 nAChR that in turn reduces microglial activation in the hippocampus. The notion regarding microglial activation is further supported by the finding is that pretreatment of TQS also reduces LPS-induced increase in hippocampal ionized calcium-binding adapter molecule 1 (Iba-1), another microglial marker, expression in mice (23), suggesting an involvement of α7 nAChR. Furthermore, microglial cells adopt resting phenotype in inactivated state but undergo rapid activation in response to acute insults. This leads to change in their morphological phenotype characterized by retracted processes, hypertrophy and amoeboid morphology under strongly pathological conditions (17,22,26). Our results show that TQS-mediated antiallodynic and antihyperalgesic effects (23) are due to reduced microglial activation as indicated by reduced morphological changes in the hippocampus. It is important to mention that active NF-κB within the microglia increases expression of proinflammatory cytokines and other mediators through NF-κB mediated gene expression that plays a critical role in pain facilitation (6, 27). Therefore, further studies are essential to examine the involvement of proinflammatory cytokines and other mediators, including brain-derived neurotrophic factor during LPS-induced hyperalgesia and allodynia in the hippocampus.
In conclusion, this study demonstrated that α7 nAChR PAM decreases IκB and CD11b gene expression and microglial activation associated with hyperalgesia and allodynia by targeting microglial α7 nAChR in the hippocampus. Therefore, α7 nAChR PAM may represent a new class of treatment for neuroinflammatory pain.
Acknowledgements
This study was supported by Fulbright Foundation, USA (MA and SR) and SDSU Research Foundation (SR). RLP is supported by NIH grant GM57481.
References
- 1.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: Native subtypes and their relevance. Trends Pharmacol Sci. 2006; 27:482–491. [DOI] [PubMed] [Google Scholar]
- 2.Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007; 30:527–535. [DOI] [PubMed] [Google Scholar]
- 3.Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by alpha7 nicotinic receptors. J Neurochem. 2004; 89:337–343. [DOI] [PubMed] [Google Scholar]
- 4.Alsharari SD, Freitas K, Damaj MI. Functional role of alpha7 nicotinic receptor in chronic neuropathic and inflammatory pain: Studies in transgenic mice. Biochem Pharmacol. 2013; 86:1201–1207. [DOI] [PubMed] [Google Scholar]
- 5.Feuerbach D, Lingenhoehl K, Olpe HR, Vassout A, Gentsch C, Chaperon F, Nozulak J, Enz A, Bilbe G, McAllister K, Hoyer D. The selective nicotinic acetylcholine receptor alpha7 agonist JN403 is active in animal models of cognition, sensory gating, epilepsy and pain. Neuropharmacology. 2009; 56:254–263. [DOI] [PubMed] [Google Scholar]
- 6.Loram LC, Harrison JA, Chao L, Taylor FR, Reddy A, Travis CL, Giffard R, Al-Abed Y, Tracey K, Maier SF. Intrathecal injection of an alpha seven nicotinic acetylcholine receptor agonist attenuates gp120-induced mechanical allodynia and spinal pro-inflammatory cytokine profiles in rats. Brain Behav Immun. 2010; 24:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papke RL, Kem WR, Soti F, Lopez-Hernandez GY, Horenstein NA. Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines and nicotine. J Pharmacol Exp Ther. 2009; 329:791–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams DK, Wang J, Papke RL. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: Advantages and limitations. Biochem Pharmacol. 2011; 82:915–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gronlien JH, Hakerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, Malysz J. Distinct profiles of alpha7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol. 2007; 72:715–724. [DOI] [PubMed] [Google Scholar]
- 10.Bagdas D, Wilkerson JL, Kulkarni A, Toma W, AlSharari S, Gul Z, Lichtman AH, Papke RL, Thakur GA, Damaj MI. The alpha7 nicotinic receptor dual allosteric agonist and positive allosteric modulator GAT107 reverses nociception in mouse models of inflammatory and neuropathic pain. Br J Pharmacol. 2016; 173:2506–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freitas K, Negus SS, Carroll FI, Damaj MI. In vivo pharmacological interactions between a type II positive allosteric modulator of alpha7 nicotinic ACh receptors and nicotinic agonists in a murine tonic pain model. Br J Pharmacol. 2013; 169:567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khanna S, Sinclair JG. Noxious stimuli produce prolonged changes in the CA1 region of the rat hippocampus. Pain. 1989; 39:337–343. [DOI] [PubMed] [Google Scholar]
- 13.Soleimannejad E, Naghdi N, Semnanian S, Fathollahi Y, Kazemnejad A. Antinociceptive effect of intrahippocampal CA1 and dentate gyrus injection of MK801 and AP5 in the formalin test in adult male rats. Eur J Pharmacol. 2007; 562:39–46. [DOI] [PubMed] [Google Scholar]
- 14.Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990; 39:151–170. [DOI] [PubMed] [Google Scholar]
- 15.Watkins LR, Milligan ED, Maier SF. Glial activation: A driving force for pathological pain. Trends Neurosci. 2001; 24:450–455. [DOI] [PubMed] [Google Scholar]
- 16.DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996; 16:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverman HA, Dancho M, Regnier-Golanov A, Nasim M, Ochani M, Olofsson PS, Ahmed M, Miller EJ, Chavan SS, Golanov E, Metz CN, Tracey KJ, Pavlov VA. Brain region-specific alterations in the gene expression of cytokines, immune cell markers and cholinergic system components during peripheral endotoxin-induced inflammation. Mol Med. 2015; 20:601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Y, Lecker S, Post MJ, Hietaranta AJ, Li J, Volk R, Li M, Sato K, Saluja AK, Steer ML, Goldberg AL, Simons M. Inhibition of ubiquitin-proteasome pathway-mediated I kappa B alpha degradation by a naturally occurring antibacterial peptide. J Clin Invest. 2000; 106:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nature Rev Immunol. 2002; 2:725–734. [DOI] [PubMed] [Google Scholar]
- 20.Sun SC, Ganchi PA, Ballard DW, Greene WC. NF-kappa B controls expression of inhibitor I kappa B alpha: Evidence for an inducible autoregulatory pathway. Science. 1993; 259:1912–1915. [DOI] [PubMed] [Google Scholar]
- 21.Yoshikawa H, Kurokawa M, Ozaki N, Nara K, Atou K, Takada E, Kamochi H, Suzuki N. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin Exp Immunol. 2006; 146:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: A big problem from molecules in “small” glia. Trends Neurosci. 2005; 28:101–107. [DOI] [PubMed] [Google Scholar]
- 23.Abbas M, Rahman S. Effects of alpha-7 nicotinic acetylcholine receptor positive allosteric modulator on lipopolysaccharide-induced neuroinflammatory pain in mice. Eur J Pharmacol. 2016; 783:85–91. [DOI] [PubMed] [Google Scholar]
- 24.Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP. Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci. 1998; 18:3251–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma W, Bisby MA. Increased activation of nuclear factor kappa B in rat lumbar dorsal root ganglion neurons following partial sciatic nerve injuries. Brain Res. 1998; 797:243–254. [DOI] [PubMed] [Google Scholar]
- 26.Watkins LR, Maier SF. Glia: A novel drug discovery target for clinical pain. Nature Rev Drug Discov. 2003; 2:973–985. [DOI] [PubMed] [Google Scholar]
- 27.Ferrini F, De Koninck Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plasticity. 2013; 2013:429815. [DOI] [PMC free article] [PubMed] [Google Scholar]