Figure 3.
Structural Basis for the Lack of Selectivity with the High-Affinity DMF5 Mutations toward MART-1 Homologs
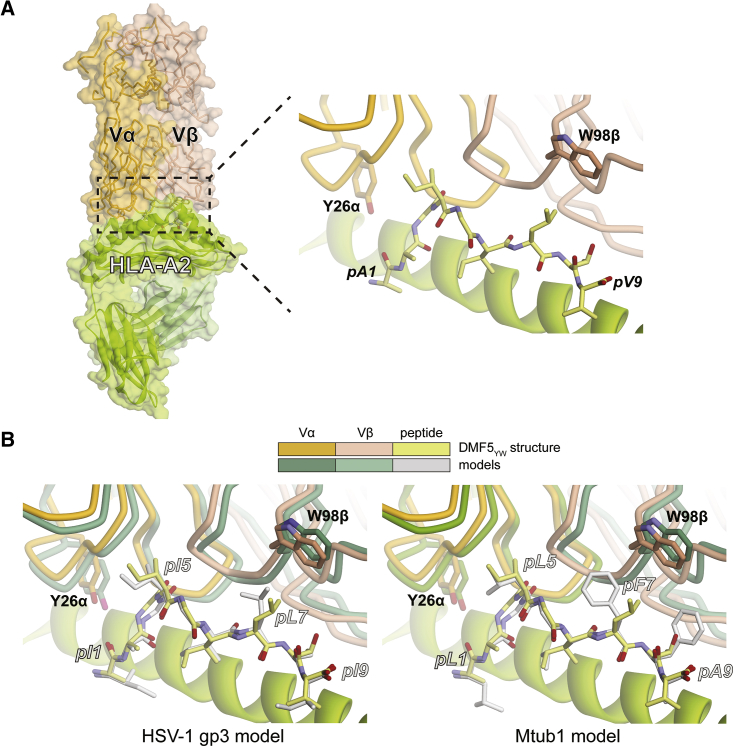
(A) Interface between the high-affinity DMF5YW variant and the MART-1 nonamer presented by HLA-A2, as revealed by the crystallographic structure of the complex. (B) Modeling of the HSV-1 gp3 and Mtub1 peptides in the interface DMF5YW forms with the MART-1 nonamer presented by HLA-A2 interface suggests there are no requirements for significant conformational changes and that the environments around the high-affinity mutations are conserved.