Figure 5.
Implementation of a Positive and Negative Design Strategy in the DMF5 TCR
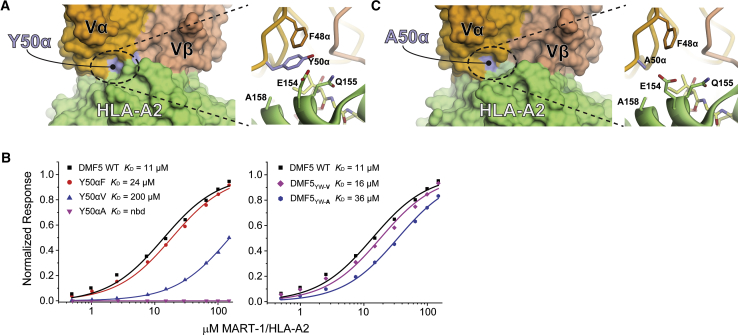
(A) Tyr50 of the DMF5 CDR2α loop interfaces with the HLA-A2 α2 helix, contacting a stretch of amino acids from Gln155 to Ala158. (B) Mutating Tyr50α to phenylalanine, valine, and alanine progressively weakens binding of DMF5 to the MART-1 decamer presented by HLA-A2. nbd, no binding detected (left panel). Combining the Y50αV and Y50αA mutations with the peptide-centric D26αY and L98βW mutations that lead to high-affinity binding, creating the DMF5YW-V and DMF5YW-A triple mutants, returns binding to levels slightly weaker than wild-type (right panel). (C) Mutating Tyr50α to alanine has no gross structural consequences, as shown by the structure of DMF5YW-A bound to the MART-1 decamer presented by HLA-A2.