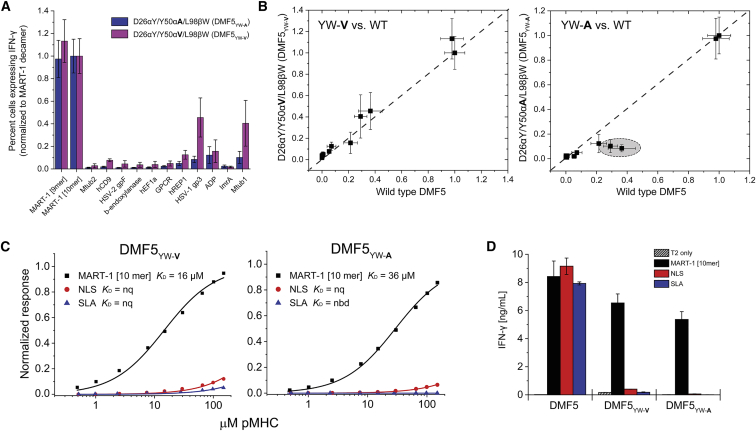
Figure 6.
DMF5 Variants Incorporating Positive and Negative Design Principles Show Reduced Cross-Reactivity Compared to High-Affinity and Wild-Type TCR
(A) Percent transduced CD8+ T cells expressing intracellular IFN-γ after co-culturing TCR-transduced PBMCs with T2 cells pulsed with the MART-1 peptides and each of the MART-1 homologs in Table 1. Data are averages of six sets of experiments (two independent repeats from three donors), normalized to the values for the MART-1 decamer. Error bars indicate SEM. (B) Direct comparison between the T cell function of wild-type DMF5 and DMF5YW-V and DMF5YW-A, using the data from (A) and Figure 2A. Although the patterns of reactivity are similar, the DMF5YW-A mutant is slightly less cross-reactive than the DMF5YW-V mutant (circled region, right panel). (C) DMF5YW-A and DMF5YW-V have substantially reduced binding affinities toward the NLS and SLA peptides presented by HLA-A2, as measured by surface plasmon resonance. nq, binding detectable, but too weak to quantify; nbd, no binding detected. (D) Consistent with the binding experiments, functional recognition of the NLS and SLA peptides is eliminated with DMF5YW-A and DMF5YW-V, although potency toward the MART-1 decamer is slightly weaker than with wild-type DMF5. Experiments show IFN-γ secretion when TCR-transduced PBMCs were co-cultured with T2 cells pulsed with peptide. Values are averages of triplicate wells; error bars indicate SDs.