Table 1. Comparison of the H2O2 Formation Rates of Selected Acridine Derivatives in the Photochemical Oxidation of NADHa.
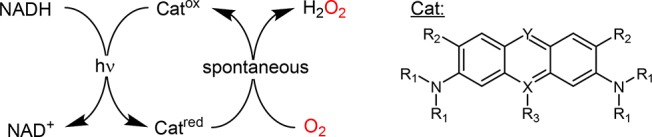
| catalyst | λmax/λex [nm]b | TF [h–1]c |
|---|---|---|
| acridine orange (R1 = CH3, R2 = H, X = N, Y = CH) | 480/450 | 66 ± 3 |
| proflavin (R1 = H, R2 = H, X = N, Y = CH) | 445/450 | 207 ± 17 |
| methylene blue (R1 = CH3, R2 = H, X = S+, Y = N) | 664 and 613shoulder/662 | 95 ± 3 |
| phenosafranine (R1 = H, R2 = H, R3 = phenyl, X = N+, Y = N) | 522/512 | 99 ± 2 |
| Safranine O (R1 = H, R2 = CH3, R3 = phenyl, X = N+, Y = N) | 507/519 | 97 ± 3 |
| FMN | 450/450 | 154 ± 18 |
General conditions: 50 μM catalysts, 50 mM KPi, pH 7.0, 1 mM NADH, 30 °C, 300 rpm.
λmax = wavelength exhibiting the maximal photoabsortion; λex = wavelength of the LED light source used for photoexcitation.
TF = turnover frequency of the catalyst = (H2O2 formation rate) [mM h–1]/(concentration of the photocatalyst) [mM].
