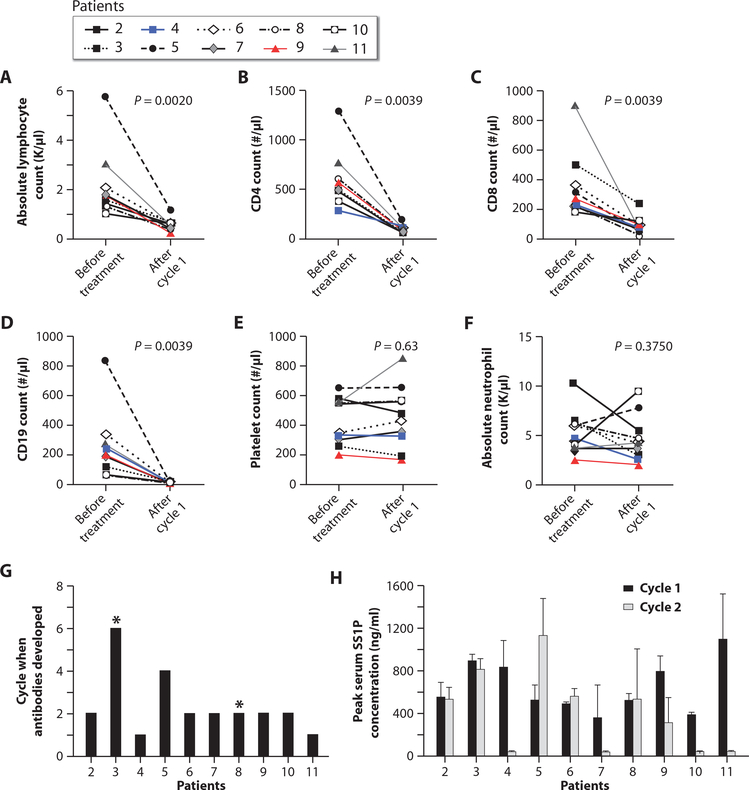
Fig. 8. Blood counts, lymphocyte subsets, SS1P neutralizing antibodies, and serum SS1P concentrations.
(A to F) Effects of pentostatin and cyclophosphamide on ALC, CD4+ T cells, CD8+ T cells, CD19+ B cells, platelets, and ANC before treatment and at the end of cycle 1 (day 30). (G) The number of treatment cycles after which each patient developed anti-SS1P neutralizing antibodies. Asterisks indicate patients who did not develop antibodies. (H) Peak serum SS1P concentrations for each patient during cycles 1 and 2, calculated as mean serum SS1P concentration on days 1, 3, and 5 of each cycle. All patients had an SS1P serum concentration of >100 ng/ml during cycle 1. However, during cycle 2, four patients did not have an SS1P serum concentration of >100 ng/ml. Error bars indicate SD.