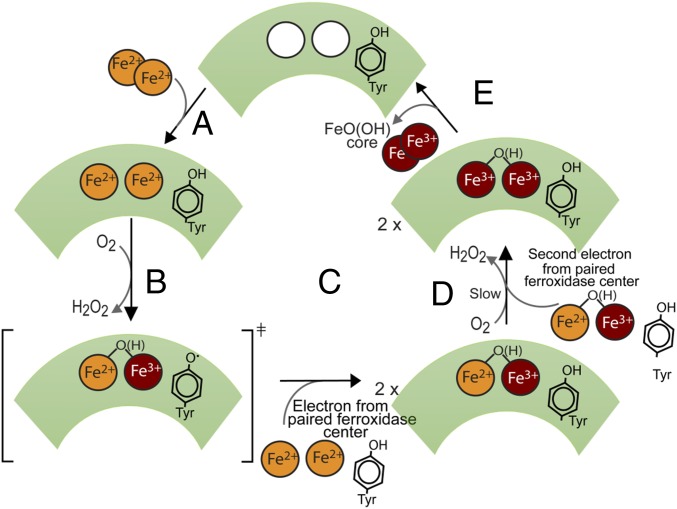
Fig. 7.
Proposed catalytic cycle of SynFtn. (A) Apo ferroxidase center binds two Fe2+ ions from solution. (B) Two-electron reduction of O2 to hydrogen peroxide leads to oxidation of a single Fe2+ ion, thus yielding the MVFC, and a radical on Tyr40. (C) The Tyr radical is transient and only observed in the subset of subunits where the partner subunit’s ferroxidase center is unoccupied. In the remainder, rapid electron transfer from Fe2+/Fe2+ or monomeric Fe2+ bound at a second ferroxidase center results in quenching of the radical. (D) Slow reaction of the MVFC with a second molecule of O2, accompanied by transfer of a second electron from the paired ferroxidase center, results in formation of the unstable bridged diferric center observed in other ferritins and a second molecule of hydrogen peroxide. The kinetics of this process was unaltered in a Y40F variant, suggesting that Tyr40 is not required for transfer of the second electron. (E) Hydration and translocation of oxidized iron from the ferroxidase center to the internal cavity results in formation of mineral core and regeneration of apo ferroxidase centers. Overall, four ferroxidase center-bound Fe2+ ions are oxidized, reducing two molecules of O2 to H2O2, accounting for the observed 2:1 stoichiometry.