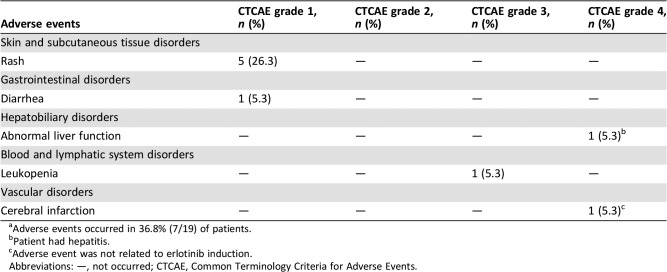
Table 5. Adverse events during erlotinib therapya .
Adverse events occurred in 36.8% (7/19) of patients.
Patient had hepatitis.
Adverse event was not related to erlotinib induction.
Abbreviations: —, not occurred; CTCAE, Common Terminology Criteria for Adverse Events.