Abstract
The chronic inflammation and damage to the gastric epithelium induced by Helicobacter pylori (H. pylori) are the main risk factors for gastric cancer development. Epstein-Barr virus (EBV) and human cytomegalovirus (HCMV) induce chronic inflammation and have been found in gastric tumors. The objectives this observational study were to determine the frequency of multiple infections by Helicobacter pylori, Epstein-Barr virus (EBV) and human cytomegalovirus (HCMV) and to relate the infection by EBV and HCMV with H. pylori vacA/cagA genotypes in patients with chronic gastritis or gastric cancer. DNA from H. pylori, EBV and HCMV was detected by PCR in biopsies from 106 Mexican patients with chronic gastritis and 32 from gastric cancer. The cagA status and the vacA genotypes of H. pylori were determined by PCR. In chronic gastritis and gastric cancer EBV was found in 69.8% and 87.5%, HCMV in 52.8% and 53.1%, and H. pylori in 48.1% and 40.6%, respectively. In chronic gastritis, 53% of H. pylori+ patients were EBV+ and 33% were both EBV+/HCMV+; in gastric cancer, 92.3% of H. pylori-infected individuals were EBV+ and 46.1% were EVB+/HCMV+. All the intestinal- and mixed-type tumors and the 83.3% of diffuse-type tumors were EBV+. No significant differences were found between single infections or coinfections with the diagnosis or the cancer type. The H. pylori genotypes were not related to EBV or HCMV infection. The frequency of dual infections by H. pylori, EBV and HCMV is higher in patients from southwest Mexico than other populations. It is likely that these pathogens act synergistically to induce inflammation and gastric cancer.
Keywords: chronic gastritis and gastric cancer, epstein-barr virus, Helicobacter pylori, human cytomegalovirus
1. Introduction
In 2013, gastric cancer was ranked third in incidence and mortality in developing countries.[1,2] In the United States of America, the gastric cancer incidence rate on Hispanic men was 73% higher than for non-Hispanic white men; among the Hispanic woman, this rate was more than twice than that among non-Hispanic white woman.[3] Based on Lauren's classification, the most common histologic types of gastric adenocarcinoma are the intestinal-type and the diffuse-type, which differ in the etiology and carcinogenic pathways.[4] Intestinal-type gastric cancer initiates inflammatory changes and focal necrosis that evolve into chronic gastritis, atrophic chronic gastritis, intestinal metaplasia, dysplasia and cancer.[5,6] On the other hand, the histologic changes that occur during diffuse carcinoma progression are still unknown, which starts with gastritis throughout the stomach but is not atrophic.[7]
Gastric cancer has a recognized infectious etiology. Chronic infection with Helicobacter pylori (H. pylori) is the risk factor that contributes the most to gastric carcinogenesis, although only 5% of infected individuals develops the disease.[3]H. pylori can induce gastric carcinogenesis by 2 main mechanisms:
-
1.
by indirect effects, stimulating a local chronic inflammatory response and
-
2.
by direct effects on the molecular composition of epithelial gastric cells through the toxic action of its virulence factors.[8]
H. pylori infection stimulates a chronic inflammatory response that leads to an increased cellular turnover that may result in the accumulation of mitotic errors. Moreover, persistent inflammation leads to atrophic gastritis, which is accompanied by hypochlorhydria or achlorhydria and the rise of the gastric pH. The clinical result depends on the severity and distribution of gastritis as well as deficiency in gastric acid secretion.[9] Through the virulence factors CagA and VacA, H. pylori directly promotes mutations in cell cycle regulatory genes, deficiencies in DNA repair mechanisms, loss of cell adhesiveness and epigenetic changes that can alter cell function and promote cell autonomy and malignant transformation.[8,10] Only a portion of the H. pylori strains possess the cagA gene but all encode the vacA gene. The latter is a polymorphic gene and the strains encoding the allelic combination s1m1 are associated with pre-neoplastic gastric lesions and cancer. The majority of the cagA+ strains are vacA s1m1.[11]
Epstein-Barr virus (EBV) can infect the superficial epithelium of the stomach through B cells carrying reactivated EBV, which may initiate carcinogenesis.[12,13] The EBV was classified as a type I carcinogenic by the International Agency for Research on Cancer (IARC) and growing evidence associates EBV with gastric cancer.[14,15] Results from in vitro studies suggest that B cells infected with EBV generate a high rate of infectiousness on epithelial cells.[16] EBV can induce gastric epithelial cell death or persist as a latent infection and promote cancer progression.[17] Some gastric carcinomas harbor the EBV monoclonal genome in each cancer cell; this finding suggests that all those cells originated from the same infected progenitor cell. The viral monoclonality in EBV-positive gastric cancer strengthens the causal relationship between EBV and gastric carcinogenesis.[18–20] Additionally, EBV-positive gastric cancer samples are characterized by recurrent mutations of PIK3CA, DNA hypermethylation and JAK2, CD274 and PDCD1LG2 amplification, which may be mechanisms involved in carcinogenesis.[17,20] It is likely that EBV exerts its oncogenic effects through nuclear antigens (EBNAs) and latent membrane proteins (LMPs), that interact with signaling pathways and tumor suppressor genes.[15] Based on the molecular characteristics that differentiate gastric tumors, the Cancer Genome Atlas Investigation Network defined EBV-associated gastric cancer as a specific subtype.[21]
In adulthood, approximately 50% of the population is infected with H. pylori and up to 95% has EBV in a latent status. Both pathogens are acquired during childhood.[16] The frequency and distribution of gastric cancer subtypes and the frequency of H. pylori and EBV differ between geographical regions of the world.[21] The EBV percentage of infection in gastric cancer varies between 4.3% and 18%.[19] The EBNA-1 protein from EBV has not been detected in healthy subjects, however, it has been found in 4.1% to 37.3% of patients with non-ulcerous gastric disease, in 16.7% to 75.6% of individuals with ulcerous peptic disease and in 80% to 90% of gastric cancer cases.[20]
Human cytomegalovirus (HCMV) is a herpesvirus that causes serious intrauterine infections as well as acute and chronic complications in immunocompromised patients.[22] HCMV is not considered oncogenic, in spite of in vitro evidence that suggests that it can cause fibroblast transformation in culture.[23] HCMV encodes approximately 185 genes from which UL133, UL135, and UL136 have been associated with gastric cancer. The UL33 expression rate in cancerous tissue is significantly higher than in normal tissue[24] and the number of gastrointestinal disease cases caused by HCMV has increased, between them are ulcerative colitis and esophageal ulcers.[25] There are still few studies documenting the frequency of HCMV infection in gastric diseases and it is still necessary to clarify the relationship of this virus with gastric cancer.
Gastric cancer incidence remains highly common in Mexico, Central and South America, and Asia but has substantially declined in high-income countries. Meanwhile, H. pylori prevalence is higher in low-income countries and among individuals with low socioeconomic status.[26] There are few studies on the presence of EBV in digestive pathologies and it is likely that the importance of this virus in gastric carcinogenesis is being underestimated. EBV infection in gastric mucosa of patients with chronic gastritis and gastric cancer is a practically unexplored field in Mexico and only 2 studies have addressed EBV and H. pylori coinfection through quantification of specific antibodies.[27,28] In this study, we estimated the frequency of single infections and coinfections by EBV, HCMV and H. pylori in patients with chronic gastritis or gastric cancer. In addition, we determined the prevalence of H. pylori vacA/cagA genotypes and evaluated the relation between the viruses and H. pylori-specific genotypes. Lastly, we analyzed the frequency of each pathogen on dual and triple infections by cancer type.
2. Materials and methods
2.1. Patients
A cross-sectional study was carried out in 138 patients, consecutively selected among those presenting symptoms of dyspepsia or presumptive diagnosis of gastric cancer. One hundred six underwent upper gastrointestinal endoscopy in the Specialized Unit in Gastroenterology and Endoscopy in Chilpancingo, Guerrero, Mexico. Thirty-two subjects were subjected to an endoscopic study to confirm gastric cancer in the Cancerology State Institute from Acapulco, Guerrero, Mexico. Patients without antimicrobial treatment and without consumption of proton pump inhibitors or gastric pH neutralizers during the 2 weeks prior to the endoscopic study were included. Patients under non-steroidal anti-inflammatory treatment or treated with immunosuppressors were excluded. Selection of patients was carried out from March 2012 to May 2015. The patients or their parents signed a written informed consent form and they were treated in accordance with the tenets of the Declaration of Helsinki. The study was approved by the Bioethics committee from from Autonomous University of Guerrero and by the Investigation Department of Cancerology State Institute.
2.2. Tissue obtainment and histological study
The endoscopic study was carried out after 1 night of fasting with a video processor and video gastroscope (Fujinon, Wayne, NJ). Two biopsies of antrum or body were taken from chronic gastritis patients and 2 tumor biopsies were taken from gastric cancer patients. A biopsy from each site was immediately fixed in 10% formalin for histological examination and another was placed in a buffer solution (Tris 10 mM pH 8.0, EDTA 20 mM pH 8.0, SDS 0.5%) in order to obtain total DNA for H. pylori, EBV and HCMV diagnosis. Biopsies for H. pylori, EBV and HCMV detection were maintained at −20°C until processing and formalin-fixed biopsies were embedded in paraffin. Four-micrometer sections of paraffin-embedded tissue were stained with hematoxylin-eosin for the histological study. The histopathological diagnosis was carried out according to the updated Sydney system[29] or based on the Padova international classification for gastric dysplasia.[30] Endoscopic and histopathological findings were only used to establish the diagnosis of the patients.
2.3. H. pylori detection and vacA genotyping
Total DNA from gastric biopsies was extracted by the Phenol-Chloroform method, after digestion with Proteinase K.[31]H. pylori DNA was detected using the primers HPF 5′-GCT AAG AGA TCA GCC TAT GTC C-3′ and HPR 5′-CAA TCA GCG TCA GTA ATG TTC-3′, modified from those used by Chang et al, which amplify a fragment of the rRNA 16S gene.[32] DNA integrity was verified adding a set of primers specific for IL-1B gene in the PCR for H. pylori 16S rRNA gene. IL-1B primers sequences were sense 5′-CAT TTG TCA GGT TCT TGA TC-3′ and antisense 5′-GAA GTT TAG TCT TCC CAC TT-3′ which amplified a 305 bp fragment. The signal and middle regions of vacA were genotyped by PCR, using the oligonucleotides previously used by Koehler et al[33] and following the methodology described by Román-Román et al.[34]
2.4. cagA detection
cagA detection was performed in H. pylori rRNA 16S positive samples by PCR, using the primers cagAF 5′-ATA ATG CTA AAT TAG ACA ACT TGA GCG A-3′ and cagAR 5′-TTA GAA TAA TCA ACA AAC ATC ACG CCA T-3′,[35] which amplify a 297 bp fragment from constant region. In addition, a second set of primers was used in order to amplify a 500 to 850 bp fragment from the 3′ cagA variable region cag2F 5′- GGA ACC CTA GTC GGT AAT G-3′ and cag4R 5′-ATC TTT GAG CTT GTC TAT CG-3′. PCR reactions were carried out in a total volume of 25 μL containing 1.7 mM MgCl2, 0.2 mM dNTP's (Invitrogen, Carlsbad, CA), 5 pmol from each primer, 1 U Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA) and 300 ng total DNA. Cycling conditions were as follows: 1 cycle at 94°C for 5 min, 30 cycles at 94°C for 40 s, 56°C for 30 s and 72°C for 50 s, with a final extension cycle at 72°C for 10 min. The resulting PCR products were resolved in 1.5% agarose gels, stained with ethidium bromide and observed under ultraviolet light (UV). Samples were considered cagA-positive when either one or both PCR products were observed.
In all PCR reactions, DNA from strain ATCC 43504 H. pylori vacA s1m1/cagA+/babA2+ was used as a positive control and the template DNA was substituted with sterile deionized water in the negative control. Total DNA from a vacA s2/m2 positive gastric biopsy was used as a positive control for s2 and m2 allele genotyping. All PCR reactions were run in a Mastercycler EP gradient thermocycler (Eppendorf, Hamburg, Germany).
2.5. EBV and HCMV detection
DNA from EBV was identified using the set of primers EBNA1-F 5′-AGG CCA TTT TTC CAC CCT G-3′ and EBNA1-R 5′- CTT CTC CTG GGT CAT CTG CG-3′, which amplify a fragment of 114 bp from the EBNA1 gene. Detection of HCMV DNA was performed using the set of primers forward UL44-F 5′-GTG CGC TCA AGG AGA ACA C-3′ and reverse UL44-R 5′-TGC ACG TAG AAC TTG GTC AG-3′, that amplify a 181 bp region from the UL44 gene. PCR reactions were performed in a total volume of 20 μL containing 200 ng DNA, 4 mM MgCl2, 0.2 mM dNTP's, 5 pmol of each primer for EBNA1, 0.4 μM of each primer for UL44 and 1 U Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA). All PCR reactions were run in a Mastercycler EP gradient thermocycler (Eppendorf, Hamburg, Germany). Cycling conditions included 1 cycle of initial denaturation at 94°C for 5 min, followed by 40 cycles of 94°C for 20 s, 57°C for 20 s for EBNA1 gene (58°C for UL44), 72°C for 30 s and a final extension cycle at 72°C for 7 min. All PCR products were resolved in 2.5% agarose gels, stained with ethidium bromide and visualized under UV light. Samples were considered positive for EBV or HCMV when the PCR products of the corresponding sizes were identified. As a positive control, an EBV+/HCMV+ sample was used. The specificity of viral DNA in the sample was verified by sequencing.
2.6. Statistical analysis
The frequencies of qualitative variables and the mean ± standard deviation (SD) of parametric quantitative variables were determined. The X2 test or the Fisher exact test were used for frequency comparisons between groups and Student's t test was used for mean comparisons. A P value of < .05 was considered statistically significant. All the statistical calculations were performed using Stata V.9.2 software (College Station, TX).
3. Results
3.1. Patients
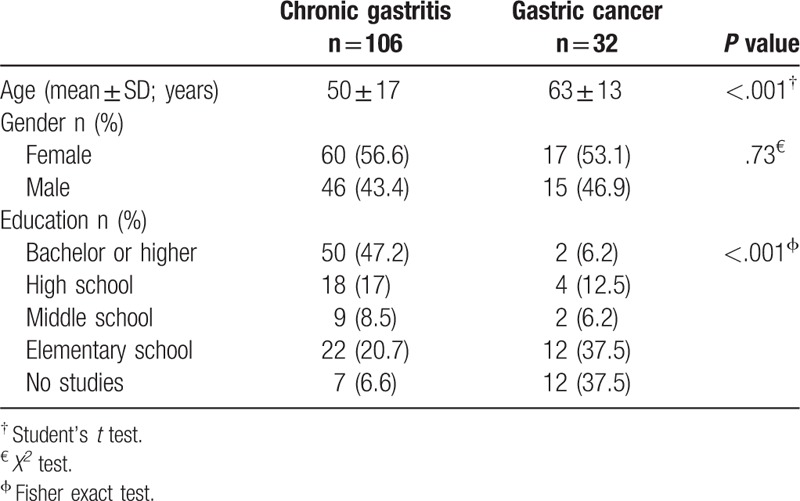
From the 138 patients included in the study, 106 (76.8%) had histopathological diagnosis of chronic gastritis and 32 (23.2%) of gastric cancer, from which 7 (21.9%) were intestinal-type, 18 (56.2%) diffuse-type, 3 (9.4%) mixed-type and 4 (12.5%) had other types of cancer. The mean age was 50 ± 17 years (4–89 years) for chronic gastritis patients and 63 ± 13 years (37–85 years) for gastric cancer patients. In both groups, the female gender predominated, 55.8% of the total patients. Groups were significantly different in age (P < .001) and educational level (P < .001), Table 1.
Table 1.
Sociodemographic features of chronic gastritis and gastric cancer patients.
3.2. EBV, HCMV and H. pylori prevalence
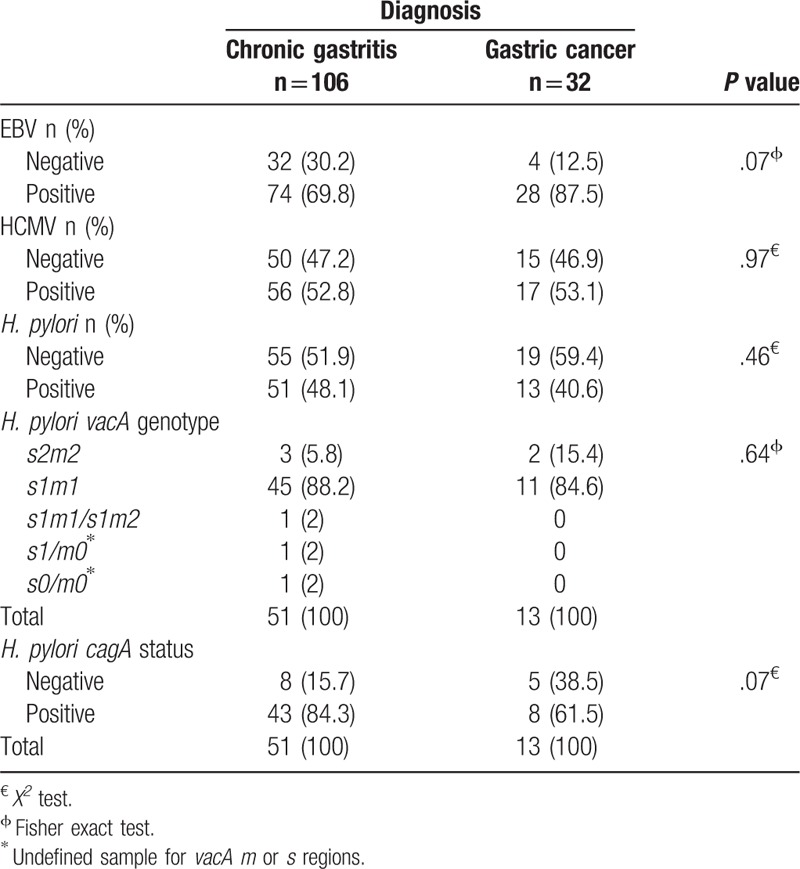
Of all patients, 73.9% (102/138) were EBV-positive, 52.9% (73/138) were HCMV-positive and 46.4% (64/138) were positive for H. pylori. EBV, HCMV and H. pylori prevalence varied according to diagnosis, Table 2. From H. pylori-positive cases, 89% (57/64) were vacA s1m1 and 79.7% (51/64) were positive for cagA. No significant differences were found in H. pylori genotype distribution between groups, Table 2.
Table 2.
EBV, HCMV and H. pylori vacA/cagA genotype frequencies in patients with chronic gastritis and gastric cancer from Guerrero state, Mexico.
3.3. EBV, HCMV and H. pylori (vacA/cagA) coinfections
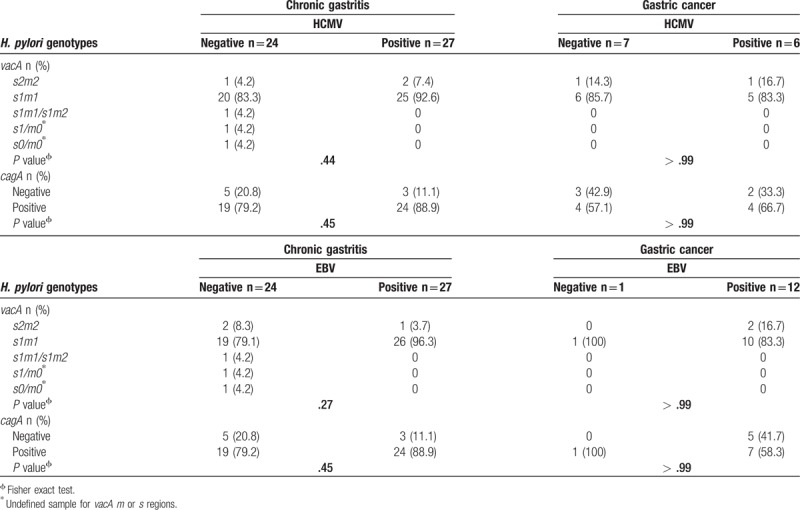
Of all patients, 41.3% (57/138) were EBV+/HCMV+, this coinfection reached 50% (16/32) in gastric cancer and 38.7% (41/106) in chronic gastritis. On the other hand, 28.3% (39/138) of patients were H. pylori+/EBV+. Prevalence of H. pylori+/EBV+ cases was higher in gastric cancer than chronic gastritis, 37.5% (12/32) and 25.5% (27/106), respectively. Of the total cases, 24% (33/138) were H. pylori+/HCMV+ and this coinfection was more frequent in the group with gastritis, 25.5% (27/106). Seventeen percent (23/138) of patients were H. pylori+/EBV+/HCMV+ and 3.6% (5/138) of patients were free from infection, Table 3. In chronic gastritis, 53% (27/51) of H. pylori+ cases were EBV+ and 33% (17/51) were EBV+/HCMV+. In gastric cancer, 92.3% (12/13) of H. pylori-infected patients were EBV+ and 46.1% (6/13) were EBV+/HCMV+, Table 3. No differences were found in EBV and HCMV distribution according to the H. pylori genotypes between groups, Table 4.
Table 3.
H. pylori, EBV and HCMV coinfection in patients with chronic gastritis and gastric cancer.
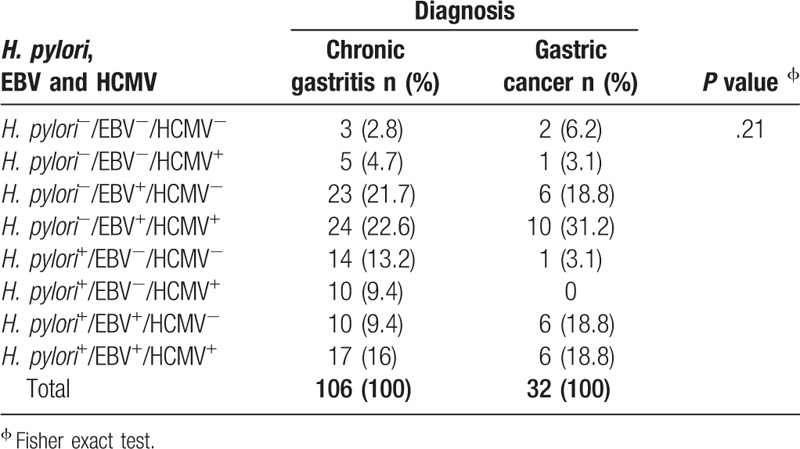
Table 4.
EBV and HCMV infection and vacA/cagA genotypes in H. pylori-positive patients with chronic gastritis or gastric cancer.
3.4. EBV, HCMV and H. pylori infection by type of cancer
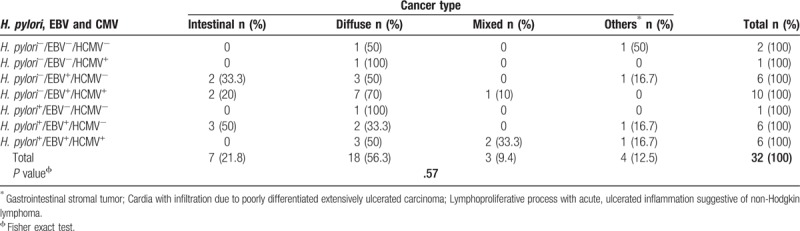
Among all cancer patients, 93.8% (30/32) were positive for at least one infectious agent. All intestinal- (7) and mixed-type (3) tumors were EBV+; in contrast, 83.3% (15/18) of diffuse-type tumors tested positive for EBV. HCMV frequency was 28.6% (2/7), 61.1% (11/18) and 100% (3/3) for intestinal-, diffuse- and mixed-type cancer, respectively. Ninety-one percent (29/32) of all tumors were EBV+, HCMV+ or EBV+/HCMV+, in 40.6% (13/32) of cases H. pylori, H. pylori/EBV or H. pylori/EBV/HCMV were found and 40% (12/30) of all infection cases were H. pylori+/EBV+. From the tumor-infected cases, 20% (6/30) were H. pylori+/EBV+/HCMV+. The 42.8% (3/7) of intestinal-type cancer and 27.7% (5/18) of diffuse-type cancer were H. pylori+/EBV+, Table 5. H. pylori infection was present in 42.8% (3/7) of intestinal-type cancer cases, in 33.3% (6/18) of diffuse-type cancer and in 66.6% (2/3) of mixed-type cancer cases.
Table 5.
EBV, HCMV and H. pylori coinfection by cancer type.
4. Discussion
Twenty percent of malignant neoplasms are induced by infectious agents. H. pylori is a type I carcinogen for gastric cancer,[36,37] nonetheless, about 50% of gastric tumors are H. pylori-negative. On the other hand, between 1.3% and 20.1% of gastric carcinomas contain EBV and the average of cases reaches 10% worldwide.[38] Some tumors are associated with HCMV.[24,39]
In this study, we analyzed EBV and HCMV frequency of infection in patients with chronic gastritis and gastric cancer with and without H. pylori infection and established the relation between the presence of a viral genome with the diagnosis of the patients and with H. pylori virulence factors. We found that the virus-H. pylori coinfections are more frequent in gastric cancer than chronic gastritis and that the EBV frequency of infection in gastritis and gastric cancer is higher than that reported in other populations.
In chronic gastritis patient's ages varied from 4 to 89 years. The youngest patients were 2 children of 4 and 6 years of age, EBV+ and EBV+/HCMV+, respectively. In gastric cancer, the minimum age was 37 years and the maximum age was 85 years. The youngest patient was diagnosed with diffuse-type gastric cancer and had a familial history of this tumor type. It is likely that this was a case of hereditary diffuse gastric cancer (HDGC), which constitutes 1% to 3% of gastric cancer cases.[40,41]
Educational level is a parameter used to evaluate the socioeconomic aspect and the marginalization level in a population.[42] In this research, 65% of gastric cancer subjects had no education or only had an elementary education. In 2016, Gómez-Dantés et al[43] reported that Guerrero is one of the states in the Mexican Republic with the highest marginalization levels and that, among Guerrero people, gastric cancer is the third cause of oncological mortality. The majority of patients included in this research come from hospitals that provide care for people with low socioeconomic status, who live in rural areas and do not have a social security program. This makes it difficult to access health services and favors gastric tumor late diagnosis, especially among people without schooling, lacking information and with scarce economic resources. In Mexico, as in most of the Latin American countries, the socioeconomic status of the population adds to the lack of a surveillance program and early detection of gastric cancer that results in high mortality from this cause.
Several authors have detected EBV genome in epithelial cells from gastric tumors and this virus has been associated with carcinogenesis. However, most of these studies have focused on cancer samples and the frequency of viral infections (EBV and HCMV) associated with H. pylori infection in chronic gastritis is unknown. We found that EBV prevalence in chronic gastritis was 69.8% and 87.5% in cancer, higher than that found in the populations of Mexico City and southeast Mexico; in the previous studies, DNA from EBV was found in 8% and 10.67% of non-atrophic gastritis and tumor tissue, respectively.[44] In children with non-atrophic gastritis from Mexico City, EBV seroprevalence was 64.3%.[27] In adults from Mexico and Paraguay with the same type of gastritis, frequency of anti-EBV IgG antibodies was 92% and in gastric cancer cases was 94.7%.[28] Ryan et al[45] found DNA from EBV in 83%, 64% and 30% of gastric tissues from Honduran patients as well as in North American adults and children with gastritis, respectively. In gastric cancer patients, the frequency of EBV DNA varies from 7.9% in Asia to 50.2% in Turkey. In gastric cancer subjects from India, 90% tested positive for EBV EBNA1 gene.[46] It has been observed that EBV frequency is geographically different.[44] The discrepancies in EBV prevalence may be explained by differences in sensibility and specificity of the methods employed between the studies, by the diagnosis of the studied patients and by the population's geographical region of origin.
Chronic inflammation is a risk factor for cancer. HCMV induces chronic gastritis and is detected in a fraction of cancer cases, so it may be that this virus is etiologically related to gastric carcinogenesis as a single infection or in synergy with EBV or H. pylori. In this research, the HCMV prevalence in gastritis and gastric cancer was 52.8% and 53.1%, respectively. There are few data on HCMV prevalence in gastritis and stomach cancer and the relation of this virus with gastric carcinogenesis is not clear yet. In developed countries, 60% of the adult population is positive for anti-HCMV IgG antibodies and in developing countries, such as Mexico, this prevalence reaches 100%. A variable percentage of people remains free of infection and may acquire HCMV at any life stage. In most of the infected individuals, the virus remains in a latent form, with a probability of reactivation and infection with a distinct strain of HCMV.[47] HCMV reactivation occurs in patients with immunosuppression of diverse etiology[48,49] and in organ donors or recipients,[50] among others. In 18 HIV-positive dyspeptic patients, Falasca et al[51] found anti-HCMV IgG antibodies but in none of them, HCMV DNA was detected in antral biopsies. On the other hand, gastritis cases caused by HCMV have been reported in immunocompetent patients[52] and gastric cancer cases that have evolved from HCMV gastritis. Interestingly, in some cases, treatment with antivirals has induced improvement in gastritis cases.[53] To our knowledge, this is the first study that determines EBV and HCMV prevalence in gastric mucosa in Mexican patients with chronic gastritis and gastric cancer with and without H. pylori infection.
In chronic gastritis patients, H. pylori frequency was 48.1%, similar to that reported by Román-Román et al[34] but smaller than to that informed by Paniagua et al[54] and Martínez-Carrillo et al[55] 40.6% of gastric cancer patients was H. pylori+, this prevalence is similar to that found in other studies in patients from Guerrero state.[34,56] It is possible that frequency variation is influenced by patient information obtainment bias. In addition, it is not possible to rule out that patients had been received antimicrobial treatment for H. pylori in the recent past. It is likely that people have used antibiotics indicated for other infections. Among the Mexican population, self-medication is a common practice, and although the sale of antibiotics without a medical prescription is prohibited in the country, Metronidazole is an antiparasitic drug that is part of the treatment scheme for H. pylori, which is an over-the-counter medication. Additionally, the probability of finding H. pylori in the biopsies decreases as structural and functional changes appear on the infected mucosa.
In patients with gastric pathology from Guerrero state, H. pylori s1m1 and cagA+ strains predominate[34] but does not explain gastritis and gastric cancer frequency. We found that in H. pylori+ patients, the s1m1 genotype predominated with a frequency of 88.2% and 84.6% in chronic gastritis and gastric cancer, respectively. The frequency of H. pylori cagA+ was 84.3% in chronic gastritis and 61.5% in cancer. The H. pylori s1m1/cagA+ genotype is recognized as the most virulent one and increases gastric cancer risk. Our observations suggest that single and dual infections with EBV and HCMV contribute to gastritis and gastric cancer etiology.
We found that 41.3% of all patients were EBV+/HCMV+ and that 16.7% were H. pylori+/EBV+/HCMV+. The most frequent pathogen was EBV in both groups of patients. In chronic gastritis, the frequency of EBV+/H.pylori+ was 25.5% and in gastric cancer was 37.5%. The prevalence of EBV and EBV/H. pylori coinfection found in this study is lower than the seroprevalence of these agents in 525 patients over 30 years of age from Mexico and Paraguay with non-atrophic gastritis, atrophic gastritis, intestinal metaplasia and cancer.[28] The differences in the age range and the diagnosis can partially determine the divergences in the prevalence of infections. The presence of EBV and H. pylori DNA is independent of the infection status and, although some EBV particles may have come from saliva, is very likely that the virus was found on infiltrating B cells and gastric epithelial cells. The detection method used prevents us from specifying the cellular source of the identified viral DNA. It has been proposed that EBV and H. pylori can act synergistically to cause and perpetuate gastritis.[28]
EBV was found in 100% of intestinal- and mixed-type tumors and in 83.3% of diffuse-type tumors. We did not find significant differences in EBV frequency by cancer type, which is in agreement with that reported by other authors.[15,28,44,46] Forty-three percent (3/7) of intestinal-type cancer cases and the 27.7% (5/18) of the diffuse-type were H. pylori+/EBV+; meanwhile, Shukla et al[46] found this dual infection in higher proportions with 58.3% and 53.8% for intestinal- and diffuse-type cancer, respectively. These differences may be due to the populations studied and to the sensibility and specificity of the employed methods.[57] The frequency of H. pylori+/EBV+ cases was higher in cancer (37.5%) than gastritis (25.5%). Our results support the hypothesis that both pathogens act in synergy to induce carcinogenesis, however, more studies addressing this matter are needed. H. pylori potentiates the inflammatory response in chronic gastritis and it drives gastric Th17 response through the HP0175 protein, a peptidyl prolyl cis, trans-isomerase (PPIase). It has been shown that HP0175 protein of H. pylori is able to elicit high production of IL-17 and IL-21 in gastric tumor infiltrating lymphocytes (TILs) and it promotes Th17 inflammation which, if prolonged and unabated, may be a condition that links the infection and gastric cancer.[58] In double and triple infections, H. pylori, EBV and HCMV can act synergistically, enhancing the expression of IL-17 and maintaining an inflammatory state that more severely damages gastric mucosa.[59–61] On the other hand, the oncoprotein CagA induces the secretion of IL-1β, IFN-ɣ, and TNF-α. IL-1β exerts its effects on gastric epithelial cells that might lead to the activation or expression of oncogenes.[58] It is known that H. pylori induces to monochloramine (NH2Cl) generation and with it the EBV reactivation.[62] Viral reactivation induces greater infiltration of B cells loaded with viral particles which increases the probability of epithelial cell infection.[45,46,62,63] Dual infection increases IL-1β, TNF-α and IL-8 production, augments immune cell infiltration and there is more damage to gastric mucosa by the effect of the infection and of a more intense chronic inflammation.[16,45,59] It has been proposed that atrophic chronic gastritis and intestinal metaplasia caused by H. pylori augments gastric epithelium susceptibility to EBV infection and that in turn, viral infection promotes carcinogenesis.[58] Meanwhile, CagA induces PLCγ activation, which favors EBV reactivation on infected B cells in vitro[64] and both agents activate the β-catenin/TCF-4 pathway, which are transforming factors in gastric cells.[16,27]
In conclusion, our results indicate that the frequency of dual and triple infections by H. pylori, EBV and HCMV — which are 3 infectious agents related to gastric carcinogenesis — is higher in patients from southwest Mexico compared to other populations. The EBV/HCMV coinfection was the most frequent in gastritis and gastric cancer patients and an important proportion of them presented H. pylori/EBV or H. pylori/HCMV coinfection suggesting the possibility of a synergic effect during chronic inflammation and carcinogenesis. The presence of EBV in malignant tumors is independent of the cancer type. It is necessary to explore the host factors and the possible interaction mechanisms between H. pylori and EBV or HCMV, as well as those between EBV and HCMV. The question that remains to resolve if H. pylori induces the EBV lytic phase and if it favors the reactivation of latent infection by HCMV.
Acknowledgments
We thank the nurses and the personnel that supported the obtainment of gastric biopsies. We thank BSc. Diana G. Soto Flores, BSc. Ana Teresa Torrijos Reyna and BSc. Damaris Aradi Damián Brito, for the support in H. pylori, EBV and HCMV DNA detection. A special thanks to Martin O. Morrugares-Ixtepan, Pathological Anatomy specialist with a subspecialty in Pathological Oncology, who was responsible for the histopathological diagnosis of some of the samples.
All the people mentioned in the acknowledgments section gave their consent for it.
Author contributions
Experimental designed, performing the virus detection and writing the paper: Oscar del Moral-Hernández.
Recruitment of patients, obtaining biopsies and histopathological diagnosis: Salomón Reyes-Navarrete, Reyes Betancourt-Linares
Organization of the database and statistical analysis: Dinorah N Martínez-Carrillo.
Analysis of data: Carlos Alberto Castañón-Sánchez, Sol de la Peña.
Experimental designed: Hilda Jiménez-Wences
Responsible of detection of H. pylori: Adolfo Román-Román.
Design and validation of primers and experiments: Daniel Hernández-Sotelo.
Experimental design, responsible of detection of H. pylori and writing the paper. Gloria Fernández-Tilapa.
All the authors revised and approved the manuscript.
Conceptualization: Oscar Del Moral-Hernández, Gloria Fernández-Tilapa.
Data curation: Dinorah N Martínez-Carrillo.
Formal analysis: Carlos Alberto Castañón-Sánchez, Dinorah N Martínez-Carrillo, Sol de la Peña.
Methodology: Oscar Del Moral-Hernández, Salomón Reyes-Navarrete, Reyes Betancourt-Linares, Hilda Jiménez-Wences, Adolfo Román-Román, Daniel Hernández-Sotelo, Gloria Fernández-Tilapa.
Supervision: Gloria Fernández-Tilapa.
Validation: Daniel Hernández-Sotelo.
Writing – original draft: Oscar Del Moral-Hernández.
Writing – review & editing: Gloria Fernández-Tilapa.
Footnotes
Abbreviations: CagA = cytotoxin-associated gene A, EBNA = nuclear antigen, EBV = Epstein Barr virus, HCMV = human citomegalovirus, HDGC = hereditary diffuse gastric cancer, IARC = International Agency for Research on Cancer, LMP = latent membrane protein, NH2Cl = monochloramine, SD = Standard deviation, VacA = vacuolating cytotoxin.
This study was carried out with funding granted by the Universidad Autónoma de Guerrero (research project 2014) and by the Secretary of Public Education of Mexico (ProDES 2014–2015). Sol de la Peña is supported by a postdoctoral fellowship awarded by the National Council of Science and Technology (CONACyT), key MOD.ORD./50/2017.
The authors declare that they have no conflicts of interests.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Fitzmaurice C, Dicker D, Pain A, et al. Global Burden of Disease Cancer Collaboration. The Global Burden of Cancer 2013. JAMA Oncol 2015;1:505–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Siegel RL, Fedewa SA, Miller KD, et al. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin 2015;65:457–80. [DOI] [PubMed] [Google Scholar]
- [4].Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand 1995;64:31–49. [DOI] [PubMed] [Google Scholar]
- [5].Correa P, Haenszel W, Cuello C, et al. A model for gastric cancer epidemiology. Lancet 1975;2:58–60. [DOI] [PubMed] [Google Scholar]
- [6].Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American cancer society award lecture on cancer epidemiology and prevention. Cancer Res 1992;52:6735–40. [PubMed] [Google Scholar]
- [7].Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest 2007;117:60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ishaq S, Nunn L. Helicobacter pylori and gastric cancer: a state of the art review. Gastroenterol Hepatol Bed Bench 2015;8:S6–14. [PMC free article] [PubMed] [Google Scholar]
- [9].Møller H, Heseltine E, Vainio H. Working group report on schistosomes, liver flukes and Helicobacter pylori. Int J Cancer 1995;60:587–9. [DOI] [PubMed] [Google Scholar]
- [10].Ohnishi N, Yuasa H, Tanaka S, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci USA 2008;105:1003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].González CA, Figueiredo C, Lic CB, et al. Helicobacter pylori cagA and vacA genotypes as predictors of progression of gastric preneoplastic lesions: a long-term follow-up in a high-risk area in Spain. Am J Gastroenterol 2011;106:867–74. [DOI] [PubMed] [Google Scholar]
- [12].Fukayama M, Hayashi Y, Iwasaki Y, et al. Epstein-Barr virus-associated gastric carcinoma and Epstein-Barr virus infection of the stomach. Lab Invest 1994;71:73–81. [PubMed] [Google Scholar]
- [13].Shannon-Lowe CD, Neuhierl B, Baldwin G, et al. Resting B cells as a transfer vehicle for Epstein-Barr virus infection of epithelial cells. Proc Natl Acad Sci USA 2006;03:7065–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].de Aquino PF, Carvalho PC, da Gama Fischer JS, et al. Epstein-Barr virus DNA associated with gastric adenocarcinoma and adjacent non-cancerous mucosa in patients from Manaus, Brazil. Genet Mol Res 2012;11:4442–6. [DOI] [PubMed] [Google Scholar]
- [15].Murphy G, Pfeiffer R, Camargo MC, et al. Meta-analysis shows that prevalence of Epstein–Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology 2009;137:824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Singh S, Jha HC. Status of epstein-barr virus coinfection with helicobacter pylori in gastric cancer. J Oncol 2017;2017:3456264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liang Q, Yao X, Tang S, et al. Integrative identification of Epstein-Barr virus-associated mutations and epigenetic alterations in gastric cancer. Gastroenterology 2014;147:1350–62. [DOI] [PubMed] [Google Scholar]
- [18].Imai S, Koizumi S, Sugiura M, et al. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc Natl Acad Sci USA 1994;91:9131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nishikawa J, Yoshiyama H, Iizasa H, et al. Epstein-Barr virus in gastric carcinoma. Cancers (Basel) 2014;6:2259–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen XZ, Chen H, Castro FA, et al. Epstein-Barr virus infection and gastric cancer: a systematic review. Medicine (Baltimore) 2015;94:e792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Network CGAR. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Britt W. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr Top Microbiol Immunol 2008;325:417–70. [DOI] [PubMed] [Google Scholar]
- [23].Michaelis M, Doerr HW, Cinatl J. The story of human cytomegalovirus and cancer: increasing evidence and open questions. Neoplasia 2009;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jin J, Hu C, Wang P, et al. Latent infection of human cytomegalovirus is associated with the development of gastric cancer. Oncol Lett 2014;8:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jang EY, Park SY, Lee EJ, et al. Diagnostic performance of the cytomegalovirus (CMV) antigenemia assay in patients with CMV gastrointestinal disease. Clin Infect Dis 2009;48:e121–4. [DOI] [PubMed] [Google Scholar]
- [26].American Cancer Society. Cancer Facts & Figures for Hispanics/Latinos 2015–2017. Atlanta: American Cancer Society; 2015. [Google Scholar]
- [27].Cárdenas-Mondragón MG, Carreón-Talavera R, Camorlinga-Ponce M, et al. Epstein Barr Virus and Helicobacter pylori co-infection are positively associated with severe gastritis in pediatric patients. PLoS One 2013;8:e62850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cárdenas-Mondragón MG, Torres J, Flores-Luna L, et al. 2015. Case–control study of Epstein–Barr virus and Helicobacter pylori serology in Latin American patients with gastric disease. Br J Cancer 2015;112:1866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stolte M, Meining A. The updated Sydney system: classification and grading of gastritis as the basis of diagnosis and treatment. Can J Gastroenterol 2001;15:591–8. [DOI] [PubMed] [Google Scholar]
- [30].Rugge M, Correa P, Dixon MF, et al. Gastric dysplasia: the Padova international classification. Am J Surg Pathol 2000;24:167–76. [DOI] [PubMed] [Google Scholar]
- [31].Sambrook J, Russell RW. Molecular cloning: a laboratory manual. 3rd edn.2001;New York, NY: Cold Spring Harbor, 2:2100. [Google Scholar]
- [32].Chang YH, Wang L, Lee MS, et al. Genotypic characterization of Helicobacter pylori cagA and vacA from biopsy specimens of patients with gastroduodenal diseases. Mt Sinai J Med 2006;73:622–6. [PubMed] [Google Scholar]
- [33].Koehler CI, Mues MB, Dienes HP, et al. Helicobacter pylori genotyping in gastric adenocarcinoma and MALT lymphoma by multiplex PCR analyses of paraffin wax embedded tissues. Mol Pathol 2003;56:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Román-Román A, Martínez-Carrillo DN, Atrisco-Morales J, et al. Helicobacter pylori vacA s1m1 genotype but not cagA or babA2 increase the risk of ulcer and gastric cancer in patients from Southern Mexico. Gut Pathog 2017;18:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Figura N, Vindigni C, Covacci A, et al. cagA positive and negative Helicobacter pylori strains are simultaneously present in the stomach of most patients with non-ulcer dyspepsia: relevance to histological damage. Gut 1998;42:772–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Compare D, Nardone G. Contribution of gut microbiota to colonic and extracolonic cancer development. Dig Dis 2011;29:554–61. [DOI] [PubMed] [Google Scholar]
- [37].Correa P. Gastric cancer: overview. Gastroenterol Clin North Am 2013;42:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen JN, He D, Tang F, et al. Epstein-Barr virus-associated gastric carcinoma: a newly defined entity. J Clin Gastroenterol 2012;46:262–71. [DOI] [PubMed] [Google Scholar]
- [39].Leila Z1, Arabzadeh SA, Afshar RM, et al. Detection of epstein-barr virus and cytomegalovirus in gastric cancers in Kerman, Iran. Asian Pac J Cancer Prev 2016;17:2423–8. [PubMed] [Google Scholar]
- [40].Pinheiro H, Oliveira C, Seruca R, et al. Hereditary diffuse gastric cancer - pathophysiology and clinical management. Best Pract Res Clin Gastroenterol 2014;28:1055–68. [DOI] [PubMed] [Google Scholar]
- [41].Tan RY, Ngeow J. Hereditary diffuse gastric cancer: what the clinician should know. World J Gastrointest Oncol 2015;7:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kweon SS, Kim MG, Kang MR, et al. Difference of stage at cancer diagnosis by socioeconomic status for four target cancers of the National Cancer Screening Program in Korea: results from the Gwangju and Jeonnam cancer registries. J Epidemiol 2017;27:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gómez-Dantés H, Lamadrid-Figueroa H, Cahuana-Hurtado L, et al. The burden of cancer in Mexico, 1990–2013. Salud Publica Mex 2016;58:118–31. [DOI] [PubMed] [Google Scholar]
- [44].Martínez-López JL, Torres J, Camorlinga-Ponce M, et al. Evidence of Epstein-Barr virus association with gastric cancer and non-atrophic gastritis. Viruses 2014;6:301–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ryan JL, Shen Y-J, Morgan DR, et al. Epstein-Barr virus infection is common in inflamed gastrointestinal mucosa. Dig Dis Sci 2012;57:1887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shukla SK, Prasad KN, Tripathi A, et al. Epstein-Barr virus DNA load and its association with Helicobacter pylori infection in gastroduodenal diseases. Braz J Infect Dis 2011;15:583–90. [PubMed] [Google Scholar]
- [47].Griffiths P, Baraniak I, Reeves M. The pathogenesis of human cytomegalovirus. J Pathol 2015;235:288–97. [DOI] [PubMed] [Google Scholar]
- [48].Mori H, Fujihara S, Nishiyama N, et al. Cytomegalovirus-associated gastric ulcer: a side effect of steroid injections for pyloric stenosis. World J Gastroenterol 2013;19:1143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mohan H1, Bal A, Garg S, et al. Cytomegalovirus-associated pseudotumor simulating gastric malignancy in acquired immunodeficiency syndrome: a case report with review of literature. Jpn J Infect Dis 2007;60:134–6. [PubMed] [Google Scholar]
- [50].Di Cocco P, Soker T, Clemente K, et al. Cytomegalovirus and gastric cancer after renal transplantation: a possible interplay. Transplant Proc 2012;44:1912–5. [DOI] [PubMed] [Google Scholar]
- [51].Falasca F, Maida P, Gaeta A, et al. Detection and quantification of EBV, HHV-6 and CMV DNA in the gastrointestinal tract of HIV-positive patients. Infection 2014;6:1033–7. [DOI] [PubMed] [Google Scholar]
- [52].Crespo P, Dias N, Marques N, et al. Gastritis as a manifestation of primary CMV infection in an immunocompetent host. BMJ Case Rep 2015. 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ueno M, Shimodate Y, Yamamoto S, et al. Gastric cancer associated with refractory cytomegalovirus gastritis. Clin J Gastroenterol 2017;10:498–502. [DOI] [PubMed] [Google Scholar]
- [54].Paniagua GL, Monroy E, Rodríguez R, et al. Frequency of vacA, cagA and babA2 virulence markers in Helicobacter pylori strains isolated from Mexican patients with chronic gastritis. Ann Clin Microbiol Antimicrob 2009;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Martínez-Carrillo DN, Garza-González E, Betancourt-Linares R, et al. Association of IL1B -511C/-31T haplotype and Helicobacter pylori vacA genotypes with gastric ulcer and chronic gastritis. BMC Gastroenterol 2010;10:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Martínez-Carrillo DN, Atrisco-Morales J, Hernández-Pando R, et al. Helicobacter pylori vacA and cagA genotype diversity and interferon gamma expression in patients with chronic gastritis and patients with gastric cancer. Rev Gastroenterol Mex 2014;79:220–8. [DOI] [PubMed] [Google Scholar]
- [57].Ribeiro J, Oliveira A, Malta M, et al. Clinical and pathological characterization of Epstein-Barr virus-associated gastric carcinomas in Portugal. World J Gastroenterol 2017;23:7292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Amedei A, Munari F, Bella CD, et al. Helicobacter pylori secreted peptidyl prolyl cis, trans-isomerase drives Th17 inflammation in gastric adenocarcinoma. Intern Emerg Med 2012;9:303–9. [DOI] [PubMed] [Google Scholar]
- [59].Li M, Boddeda SR, Chen B, et al. NK cell and Th17 responses are differentially induced in murine cytomegalovirus infected renal allografts and vary according to recipient virus dose and strain. Am J Transplant 2018;18:2647–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Afshari A, Yaghobi R, Karimi MH, et al. IL-17 mRNA expression and cytomegalovirus infection in liver transplant patients. Exp Clin Transplant 2015;1:83–9. [PubMed] [Google Scholar]
- [61].Rahal EA, Hajjar H, Rajeh M, et al. Epstein-Barr Virus and Human herpes virus 6 Type A DNA Enhance IL-17 Production in Mice. Viral Immunol 2015;28:297–302. [DOI] [PubMed] [Google Scholar]
- [62].Minoura-Etoh J, Gotoh K, Sato R, et al. Helicobacter pylori-associated oxidant monochloramine induces reactivation of Epstein-Barr virus (EBV) in gastric epithelial cells latently infected with EBV. J Med Microbiol 2006;55:905–11. [DOI] [PubMed] [Google Scholar]
- [63].de Souza CRT, de Oliveira KS, Ferraz JJS, et al. Occurrence of Helicobacter pylori and Epstein-Barr virus infection in endoscopic and gastric cancer patients from Northern Brazil. BMC Gastroenterol 2014;179:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Churin Y1, Al-Ghoul L, Kepp O, et al. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J Cell Biol 2003;161:249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]


