Extended Data Figure 8. Geometries of nucleotide-binding pockets and nucleotide-driven intrasubunit conformational changes of AAA domains.
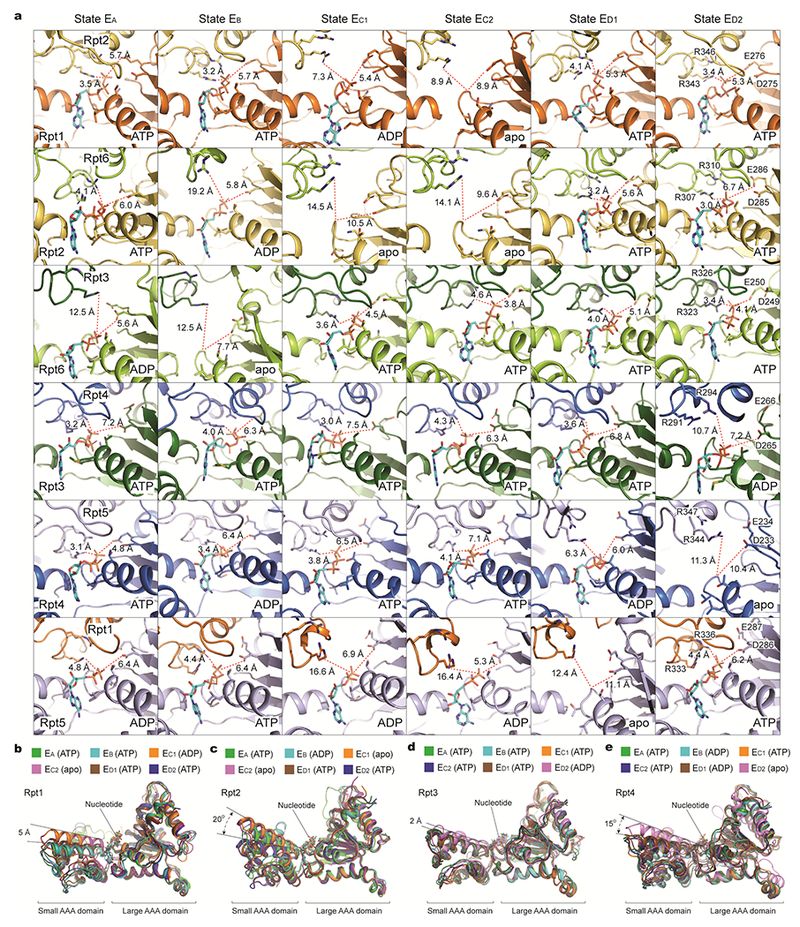
a, Comparison of the nucleotide-binding pockets of six ATPases in all states illustrates a common pattern in the geometry of the nucleotide-binding sites. Each row shows the geometry of the nucleotide-binding pocket of one ATPase in all six states. In each panel showing an ATP or ADP-bound state, one red dashed line marks the distance from β/γ-phosphate of nucleotide to the arginine finger of the adjacent ATPase, while the other line marks the distance from the same phosphate to the Walker B motif. In the case of apo-like states, the red lines extend to the proline of the Walker A motif rather than to the phosphate groups. These geometries indicate the potential reactivity of these sites49. When the ATPase is positioned in the middle of pore-loop staircase, but not at the lowest position, the nucleotide-binding pockets are tightly packed whether ATP or ADP is bound. By contrast, when the ATPase is either in the lowest position of the substrate-pore loop staircase or disengaged from the substrate, the nucleotide-binding pocket is open regardless of whether it is ADP-bound or free of nucleotide. b-e, Superposition of the AAA domain structure of Rpt1 (b), Rpt2 (c), Rpt3 (d), or Rpt4 (e) structures from six distinct states aligned against their large AAA subdomains. Rpt1 assumes two major conformations and Rpt2 assumes three.
