Extended Data Figure 9. Changes in lid-base interactions are associated with ATP hydrolysis events through long-range allosteric regulation.
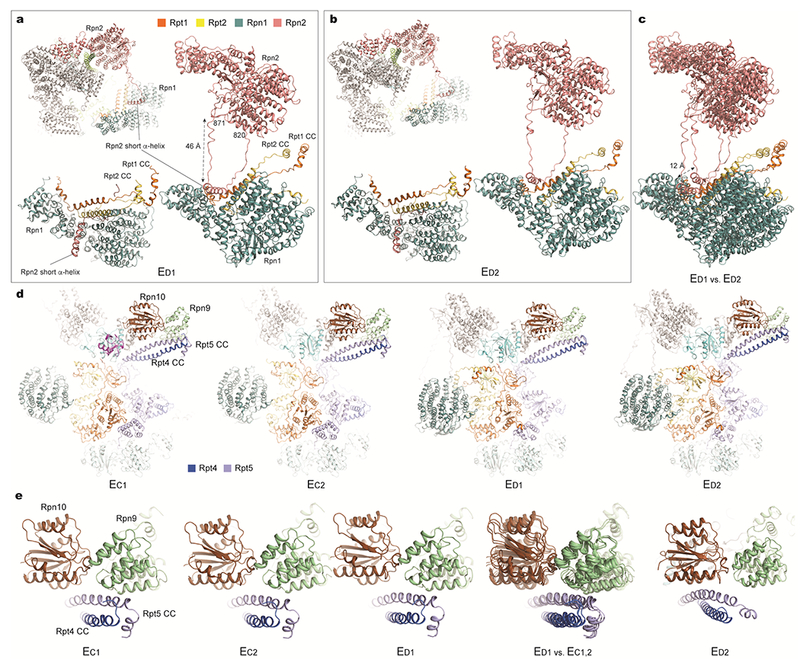
a and b, Long-range association between Rpn1 and Rpn2 through a looping structure from Rpn2 (residue 820-871) observed in state ED1 (a) and ED2 (b). c, Comparison of the Rpn1-Rpn2 long-range association between these two states shows a marked, 12 Å movement of Rpn1 relative to the CP. In both states ED1 and ED2, the Rpn1 toroidal domain and the CC domain of the Rpt1-Rpt2 dimer together form a surface cavity in which a short helix from Rpn2 is inserted50. This helix resides in the middle of a long loop (residue 820-871) emanating from the Rpn2 toroidal domain. The long-range association of Rpn1-Rpn2 seems to stabilize a larger interface formed between Rpn1-Rpn2 and Rpt1-Rpt2. However, such a quaternary architecture is not observed in other states (EA-C). In states EC1,2, the Rpn1 density is considerably blurred, reflecting strong motions potentially breaking the long-range Rpn1-Rpn2 association (Fig. 1b, Extended Data Fig. 3a). Thus, the specific Rpn1 conformation in each state appears to be highly coordinated with the hydrolytic cycle of the ATPase ring, and is controlled by Rpn1’s interactions with Rpn2 in a long-range fashion. d, Comparison of the interactions of the Rpt4-Rpt5 CC with Rpn9 and Rpn10 in states EC1,2 and ED1,3. e, Closeup views of the Rpt4-Rpt5 CC in contact with Rpn9 in states EC1,2 and ED1, and of this CC’s contact switching to Rpn10 in state ED2. These observations are consistent with a recent study51.
