ABSTRACT
Chemotherapy- or radiotherapy-related intestinal microbial dysbiosis is one of the main causes of intestinal mucositis. Cases of bacterial translocation into peripheral blood and subsequent sepsis occur as a result of dysfunction in the intestinal barrier. Evidence from recent studies depicts the characteristics of chemotherapy- or radiotherapy-related intestinal microbial dysbiosis, which creates an imbalance between beneficial and harmful bacteria in the gut. Decreases in beneficial bacteria can lead to a weakening of the resistance of the gut to harmful bacteria, resulting in robust activation of proinflammatory signaling pathways. For example, lipopolysaccharide (LPS)-producing bacteria activate the nuclear transcription factor-κB signaling pathway through binding with Toll-like receptor 4 on stressed epithelial cells, subsequently leading to secretion of proinflammatory cytokines. Nevertheless, various studies have found that the omega-3 (n–3) polyunsaturated fatty acids (PUFAs) such as docosahexaenoic acid and eicosapentaenoic acid can reverse intestinal microbial dysbiosis by increasing beneficial bacteria species, including Lactobacillus, Bifidobacterium, and butyrate-producing bacteria, such as Roseburia and Coprococcus. In addition, the n–3 PUFAs decrease the proportions of LPS-producing and mucolytic bacteria in the gut, and they can reduce inflammation as well as oxidative stress. Importantly, the n–3 PUFAs also exert anticancer effects in colorectal cancers. In this review, we summarize the characteristics of chemotherapy- or radiotherapy-related intestinal microbial dysbiosis and introduce the contributions of dysbiosis to the pathogenesis of intestinal mucositis. Next, we discuss how n–3 PUFAs could alleviate chemotherapy- or radiotherapy-related intestinal microbial dysbiosis. This review provides new insights into the clinical administration of n–3 PUFAs for the management of chemotherapy- or radiotherapy-related intestinal microbial dysbiosis.
Keywords: chemotherapy, radiotherapy, intestinal mucositis, dysbiosis, omega-3 polyunsaturated fatty acid
Introduction
Intestinal mucositis is the main lesion that develops after chemotherapy or abdominal radiotherapy (1). Both ionizing radiation and chemical reagents target the rapid renewal of crypt cells, ultimately resulting in de-epithelialization (2, 3). Patients with acute chemotherapy- or radiotherapy-induced intestinal mucositis present with symptoms including abdominal pain, vomiting, diarrhea, and digestive dysfunctions, whereas the late-onset toxicities of ionizing irradiation to the gut include fistula formation, obstruction, or even perforation (4, 5). These side effects severely affect the quality of life of these patients.
Dysbiosis denotes any change in the composition of resident commensal communities relative to the communities found in healthy individuals (6). Herein, microbial dysbiosis is characterized by a decrease in beneficial microbes, an overgrowth of harmful microbes, and a loss of microbial diversity (6). To date, it is accepted that chemotherapy or radiotherapy can cause intestinal microbial dysbiosis (7). By reviewing recent studies, Touchefeu et al. (7) reported that the intestinal microbial dysbiosis induced by chemotherapy and by abdominal radiotherapy could be characterized by decreased proportions of Clostridium cluster XIVa, Faecalibacterium prausnitzii, and Bifidobacterium and increased proportions of Enterobacteriaceae and Bacteroides. In healthy individuals, the commensal bacteria assist the hosts in improving their defense against harmful bacteria (8, 9). However, intestinal microbial dysbiosis alone is sufficient to initiate inflammation within the intestine (10). For example, LPS from Escherichia coli activates the NF-κB signaling pathway, which leads to high secretions of proinflammatory cytokines such as IL-1β, TNF-α, and IL-6 by stressed cells (11–13).
Clinically, the available drugs for treating intestinal mucositis after chemotherapy or radiotherapy include glutamine, sucralfate, and antibiotics (7). The omega-3 (n–3) PUFAs, including EPA and DHA, exhibit therapeutic potential for some autoimmune diseases such as inflammatory bowel disease and rheumatoid arthritis through their anti-inflammatory and antioxidant functions and through maintaining the integrity of the intestinal epithelium (14–17). In addition, n–3 PUFAs are able to reduce intestinal microbial dysbiosis through increasing the proportions of beneficial bacteria and decreasing the proportions of pathogenic bacteria and their products in the gut (18, 19). Moreover, results from both basic and clinical studies have confirmed the anticancer effects of n–3 PUFAs (20). On this basis, the use of n–3 PUFAs is an optional strategy for managing patients with intestinal microbial dysbiosis, especially in those patients undergoing chemotherapy or radiotherapy. In this review, we introduce the composition of the commensal microbiota in the healthy gut, after which we summarize the characteristics of chemotherapy- or radiotherapy-related intestinal microbial dysbiosis and explain the pathogenesis of mucositis associated with intestinal microbial dysbiosis. Next, by reviewing the current findings on improving beneficial gut bacteria, attenuating proinflammatory responses, and inhibiting tumor growth with n–3 PUFAs, we show the potential for managing patients experiencing chemotherapy- or radiotherapy-related intestinal microbial dysbiosis with the use of n–3 PUFAs. Taken together, we provide new insights into the clinical use of n–3 PUFAs for patients with abdominal cancer with chemotherapy- or radiotherapy-related intestinal microbial dysbiosis.
Composition of Commensal Bacteria in the Human Intestinal Tract
In healthy individuals, the number of species of commensal bacteria in the gut ranges from 500 to 1000 because the selection pressure in the gut restricts bacterial diversity (21). Within the gut, phyla including Bacteroidetes and Firmicutes account for ∼98% of intestinal microflora (21, 22). By contrast, Proteobacteria, Actinobacteria, and Fusobacteria account for <1% (23). However, gut microbe composition varies among healthy individuals (24). Nevertheless, the proportions of anaerobes are overwhelmingly superior to the aerobes in a healthy gut (25) because the microenvironment in a heathy gut is anoxic, thus benefiting the growth of anaerobes (26), such as Bacteroides and Bifidobacterium (27). For the distribution of intestinal microflora, the small intestine is dominated by Firmicutes and Actinobacteria and the colon is dominated by Bacteroidetes and the Lachnospiraceae family (28). Collectively, the above information suggests that the composition of commensal bacteria is well orchestrated in the gut.
Specific Effects of Intestinal Commensal Bacteria on Gut Homeostasis
The intestinal commensal bacteria are crucial in maintaining gut homeostasis (29). As documented, the intestinal commensal bacteria function in several ways, such as modulating nutrient metabolism and absorption, maintaining epithelial homeostasis, and improving gut immune tolerance (21, 29, 30).
The gut is the main site of food digestion, in which dietary nutrients are metabolized and absorbed. Intestinal commensal bacteria are important contributors to these processes. By taking advantage of dietary nutrients, the commensal bacteria are able to produce essential substances benefiting human health, such as vitamin B-12 (31), vitamin K-2 (32), and several essential amino acids (33). Similarly, fermentation of dietary fibers by anaerobes including Lactobacillus and butyrate-producing bacteria allows these microbes to produce SCFAs, which include acetic acid, butyric acid, and propionic acid (30, 34). The SCFAs can be consumed by enterocytes for intracellular energy production, thus facilitating the biochemical processes (30, 33–35). Moreover, the commensal bacteria participate in the metabolism of bile acids. In this case, primary bile acids can be converted into >20 different secondary bile acid metabolites, which facilitate dietary lipid turnover and absorption (36). In addition, a vegetarian diet is rich in polyphenols (37). To achieve an appropriate bioavailability of dietary polyphenols, the gut microbiota function in metabolizing such polyphenols into absorbable compounds (37). For example, equol, a metabolite of the soya isoflavone daidzein, exhibits its high affinity toward estrogen receptor (ER), thus provoking the biological effects by the interaction between equol and ER (37).
In addition to nutrient metabolism and absorption, the intestine serves a barrier function, because it is the intestinal epithelium that separates the human body from the outside environment. To avoid epithelial injury, Lactobacillus forms a biofilm covering the epithelium to separate the pathogen-associated receptors on enterocytes from harmful bacteria in the gut (38). And Streptococcus thermophiles can produce lactic acid to inhibit the growth of harmful bacteria by decreasing the pH of the intestinal tract (39). Moreover, the commensal bacteria help maintain the integrity of the intestinal epithelium. For example, Lactobacillus can stimulate the biosynthesis of heat-shock protein 72 within enterocytes in a p38 mitogen-activated protein kinase (p38/MAPK)–dependent manner, leading to an increased tolerance of enterocytes toward foreign stimuli (40). In addition, the SCFAs produced by Lactobacillus or by butyrate-producing bacteria function in improving epithelial homeostasis (30, 33–35). By taking advantage of SCFAs, the intestinal epithelial cells upregulate their expressions of genes related to cell differentiation and proliferation (33, 34). Meanwhile, upon SCFA stimulation, goblet cells can increase their mucus production and secretion (30, 33, 35). Moreover, SCFAs protect intestinal epithelial cells against oxidative stress–induced apoptosis (31). In addition to these effects, SCFAs exert several other impacts on intestinal barrier, such as the inhibition of NF-κB, activation of inflammasomes and subsequent production of IL-18, increased secretion of secretory IgA (sIgA) by B cells, and increased proportions of T-regulatory cells and tolerogenic dendritic cells in the intestine (30). In this aspect, the SCFA-producing bacteria are crucial to inducing gut immune tolerance toward lumen antigens. However, when being challenged with chemotherapy or radiotherapy, intestinal microbial dysbiosis commonly occurs (7). In this context, Lactobacillus or butyrate-producing bacteria were decreased in the gut (41, 42), thus weakening the intestinal barrier function.
Chemotherapy- or Radiotherapy-Related Intestinal Microbial Dysbiosis and Mucositis Development
Characteristics of chemotherapy- or radiotherapy-related intestinal microbial dysbiosis
The pathogenesis of intestinal mucositis after chemotherapy or radiotherapy is complicated (43, 44). Herein, chemo- therapy- or radiotherapy-related intestinal microbial dysbiosis enables proinflammatory responses within the gut to be sustained (7). Commonly, chemotherapy- or radiotherapy-related intestinal microbial dysbiosis is characterized by an imbalance in the proportions of beneficial bacteria and harmful bacteria, perhaps even presenting as an absolute dearth of beneficial bacteria and overreproduction of harmful bacteria in the gut (7). With regard to the contributions of intestinal microbial dysbiosis to the pathogenesis of mucositis, it has been shown that oral delivery of feces from enteritic mice caused germ-free mice to become predisposed to colitis induced by dextran sulfate sodium (45). Moreover, the germ-free mice were more resistant to ionizing irradiation than conventional mice (46) because turnover of the intestinal epithelium in germ-free mice is impaired due to the lack of commensal bacteria, which contribute to epithelial self-renewal in conventional mice (47). As noted above, both ionizing irradiation and chemical reagents selectively kill the expanding cells within crypts (2, 3). Alternatively, even in the case of intestinal microbial dysbiosis, several species of harmful bacteria, such as E. coli (48) and Fusobacterium (49), have been shown to stimulate epithelial turnover in conventional mice.
Clinically, remarkable alterations in the constitution of the intestinal microbiota are observed after chemotherapy or radiotherapy (Table 1). Such alterations are associated with dysfunctions of the intestinal barrier. In the intestinal microbial dysbiosis induced by abdominal radiotherapy, compared with patients without diarrhea, patients with diarrhea symptoms presented with a higher abundance of Bacteroides, Escherichia, and Megamonas in their feces (42). Likewise, a decline in the fecal proportion of members of the Firmicutes phylum is also a typical feature of intestinal microbial dysbiosis induced by chemotherapy and pelvic radiotherapy (51, 52). In addition, Wang et al. (42) determined that if the 16S ribosomal RNA ratios of Firmicutes to Bacteroides in feces were >2.15 in patients before receiving abdominal radiotherapy, it would predict that they were more susceptible to enteritis than patients with ratios <1.79, suggesting that the ratio of Firmicutes to Bacteroides could be used as an indicator of enteritis. Nevertheless, on the basis of recent data, we propose that this indicator may not be applicable to patients with long-term intake of antibiotics or those with inflammatory bowel disease due to the pre-existing intestinal microbial dysbiosis in these patients before cancer treatment (6). Moreover, the patients with diarrhea exhibited sharp elevations in serum LPS after abdominal radiotherapy as well. For the alterations in intestinal microbiota after chemotherapy, Montassier et al. (50) identified that the feces of these patients contained high proportions of Proteobacteria and Enterobacteriaceae, thus resulting in metabolic disorders of nucleotides, energy, and vitamins among these patients. In addition, among the patients with diarrhea after chemotherapy, there were decreased amounts of Lactobacillus and Bifidobacterium in the feces, with accompanying increased proportions of E. coli and Staphylococcus (41). However, from the published literature, evidence suggesting that radiotherapy can significantly alter the fecal proportions of Lactobacillus and Bifidobacterium is still unavailable (42, 52, 54).
TABLE 1.
Characteristics of chemotherapy- or radiotherapy-related dysbiosis1
| Study, year (ref) | Disease/no. of patients | Chemo- or radiotherapy | Bacteria-detecting techniques | Samples | Main findings |
|---|---|---|---|---|---|
| Wang et al., 2015 (42) | Cervical cancer/8Anal cancer/1Colorectal cancer/2 | Pelvic radiotherapy DT: 44 ∼ 50 Gy in 22 ∼ 25 fractions | 454 Pyrosequencing | Feces | • Bacterial phylum:Firmicutes vs. Bacteroidetes ratio ↓• Bacterial family: Lachnospiraceae ↓ |
| • Bacterial genus: Faecalibacterium ↓, Oscillibacter ↓, Roseburia ↓, Streptococcus ↓, Clostridium XIVa ↑, Bacteroides ↑ | |||||
| • Diarrhea vs. no diarrhea: Clostridium XIVa ↓, Sutterella ↓ | |||||
| Montassier et al., 2015 (50) | Non-Hodgkin lymphoma/28 | Chemotherapy | 454 Pyrosequencing | Feces | • Bacterial phylum: Firmicutes ↓, Actinobacteria ↓, Proteobacteria ↑ |
| • Bacterial family: Enterococcaceae ↑, Enterobacteriaceae ↑ | |||||
| • Bacterial genus: Ruminococcus ↓, Oscillospira ↓, Blautia ↓, Lachnospira ↓, Roseburia ↓, Dorea ↓, Coprococcus ↓, Anaerostipes ↓, Clostridium ↓, Collinsella ↓, Adlercreutzia ↓, Bifidobacterium ↓, Citrobacter ↑, Klebsiella ↑, Enterococcus ↑, Megasphaera ↑, Parabacteroides ↑ | |||||
| • Capacities of metabolism: energy metabolism ↓, cofactors metabolism ↓, vitamins metabolism ↓, glycan metabolism ↑, signal transduction ↑, xenobiotics biodegradation, ↑ | |||||
| Montassier et al., 2014 (51) | Non-Hodgkin lymphoma/8 | Chemotherapy | 454 Pyrosequencing | Feces | • Bacterial phylum: Firmicutes ↓, Actinobacteria ↓, Firmicutes vs. Bacteroidetes ratio ↓, Proteobacteria ↑, Bacteroidetes ↑ |
| • Bacterial genus: Blautia ↓, Faecalibacterium ↓, Roseburia ↓, Bacteroides ↑, Escherichia ↑ | |||||
| Nam et al., 2013 (52) | Gynecological cancer/9 | Pelvic radiotherapy DT: 50.4 Gy in 28 fractions | 454 Pyrosequencing | Feces | • Bacterial phylum: Firmicutes ↓, Fusobacterium ↑• Bacterial family: Eubacteriaceae ↓, Fusobacteriaceae ↑, Streptococcacea ↑ |
| Stringer et al., 2013 (41) | Colorectal cancer/11 Breast cancer/2 Melanoma/1Healthy volunteers/2 | Chemotherapy | qPCR | Feces | • Bacterial genus: Lactobacillus ↓, Bacteroides ↓, Bifidobacterium ↓, Enterococcus ↓, Staphylococcus ↑• Bacterial species: Escherichia coli ↑ |
| Zwielehner et al., 2011 (53) | Leukemia/5Myeloma/2Non-Hodgkin lymphoma/3Gastrointestinal cancer/3Breast cancer/1Thymus cancer/1Urothelial cancer/1Ovarian cancer/1 | Chemotherapy | Bacterial 16S-sequencing | Feces | • Bacterial genus: Bifidobacteria ↓, Lactobacillus ↓, Veillonella ↓, Clostridium cluster XIVa ↓, Bacteroides ↑, Clostridium cluster IV ↑• Bacterial species: Faecalibacterium prausnitzii ↑, Enterococcus faecium ↑, Clostridium difficile ↑ |
| Manichanh et al., 2008 (54) | Abdominal tumors/10Healthy controls/5 | Abdominal radiotherapy DT: 43.2∼54.0 Gy in 25 fractions | Bacterial 16S-sequencing | Feces | • Bacterial phylum: Actinobacteria ↑• Bacterial class: Bacilli ↑, Clostridia ↓ |
1DT, dose in total; ref, reference; ↓, decreased; ↑, increased.
Chemotherapy- or radiotherapy-related intestinal microbial dysbiosis provokes the proinflammatory events in the lesioned gut
The robust activation of the NF-κB signaling pathway by the interactions between the ligands of Toll-like receptors (TLRs) and TLRs on enterocytes contributes significantly to the disordered milieu present within the inflammation of a lesioned gut (55). The TLR ligands of gut microbes are recognized by the TLRs on enterocytes; in this context, downstream transcriptional factors of the NF-κB family alter their target genes, which ultimately provokes the stressed cells to produce different inflammatory cytokines (56). For example, when being challenged with chemotherapy or radiotherapy, TLR4 drives the secretions of IL-1β, TNF-α, and IL-6 by enterocytes corresponding to LPS-producing bacteria (11–13, 57). This underlying mechanism is shown in Figure 1. Recently, the specific relation between radiation-induced intestinal microbial dysbiosis and the host secretion of IL-1β was identified by Gerassy-Vainberg et al. (45). They found that 3 dominant bacterial phyla—Proteobacteria, Verrucomicrobia, and Firmicutes—had altered proportions in an irradiated gut. By using the genera classification method, Akkermansia in the Verrucomicrobia phylum was found to be abundant, as was Sutterella in the Proteobacteria phylum. Among these bacteria, the proportions of Proteobacteria and Verrucomicrobia were increased, whereas the proportion of Firmicutes was decreased in the gut. Such alterations were highly associated with an increased secretion of IL-1β by the host. Moreover, when these irradiated mice were administered their own feces orally, the concentration of IL-1β within the lesioned colonic tissue increased further (45). Moreover, some anti-inflammatory bacteria, such as F. prausnitzii and Bifidobacterium (8, 58), decreased their amounts, or even disappeared after chemotherapy (50, 59). In this context, the proinflammatory events could become robust partially due to a lack of F. prausnitzii and Bifidobacterium, which could induce host secretion of IL-10 (58) and antagonize inhibitor of κB (IκB) degradation by producing the nonlipophilic compounds (8), respectively. Similarly, Ruminococcus, Coprococcus, Dorea, and Roseburia have been reported to be capable of inhibiting the activation of the NF-κB signaling pathway among stressed enterocytes (60). If these bacteria were absent after chemotherapy or radiotherapy, intestinal inflammation potentially increased.
FIGURE 1.
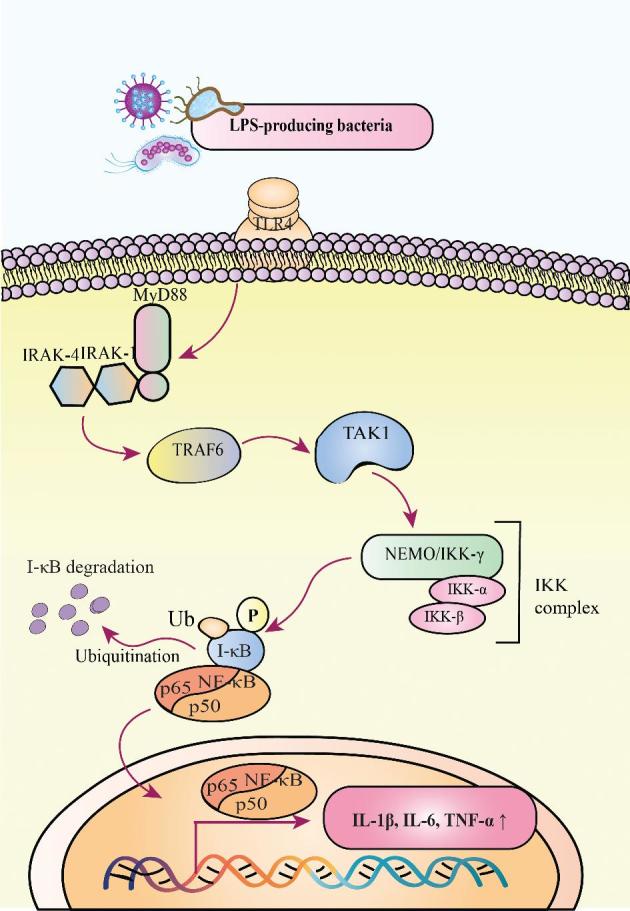
LPS-TLR4 activates the NF-κB signaling pathway. During this process, MyD88 is recruited to TLR4. Then, IRAKs and TRAF6 are activated step by step. Herein, TRAF6 activates the TAK1 molecule, which is essential for subsequent activation of the IKK complex. The IKK complex can phosphorylate the IκB molecule, which ultimately undergoes ubiquitination and degradation. Then, the NF-κB, consisting of the subunits p50 and p65, will translocate into the nucleus to initiate the transcriptions of genes encoding IL-1β, IL-6, and TNF-α. IKK, inhibitor of κB kinase; IRAK, IL-1 receptor–associated kinase; I-κB, inhibitor of κB; MyD88, myeloid differentiation factor 88; NEMO, NF-κB essential modulator; P, phosphorylation; TAK1, TGF-β–activated kinase 1; TLR4, Toll-like receptor 4; TRAF6, TNF receptor–associated factor 6; Ub, ubiquitination; ↑, increase.
Chemotherapy- or radiotherapy-related intestinal microbial dysbiosis increases oxidative stress
After chemotherapy or radiotherapy, intestinal inflammation is followed by oxidative stress, because some proinflammatory cytokines are capable of triggering the production of oxyradicals (Figure 2). For example, IL-1β is capable of inducing neutrophils to release superoxide through activation of the p38/MAPK signaling pathway (61). In addition, IL-1β could stimulate the T-helper 17 (Th17) cells to produce IL-17A (62), which would aggravate the oxidative stress through increasing endogenous secretion of granulocyte colony-stimulating factor (G-CSF) (63). Likewise, TNF-α could upregulate the expression of the gene encoding G-CSF in stressed fibroblasts (64). G-CSF is a potent neutrophil-recruiting cytokine, which could clear lesioned cells or bacterial infection by releasing reactive oxygen species (ROS) (65). Before infiltrating into lesioned sites, the circulating neutrophils must pass through the microvascular wall to reach their destination. IL-1β is also capable of upregulating the expression of the gene encoding inducible NO synthase (iNOS), thus enabling an increase in capillary permeability by promoting the production of NO within the endothelium (66). IL-6 is capable of promoting superoxide production via upregulation of the expression of the gene encoding the angiotensin II type 1 receptor on endothelial cells (67). In return, such ROS stimulate IL-6 secretion by inducing caveolin-1 to bind with Sirtuin 1 (Sirt1), therefore leading to the inactivation of Sirt1 (68). From this aspect, the inflammation and oxidative stress after chemotherapy or radiotherapy mutually aggravate the milieu within the lesioned gut.
FIGURE 2.
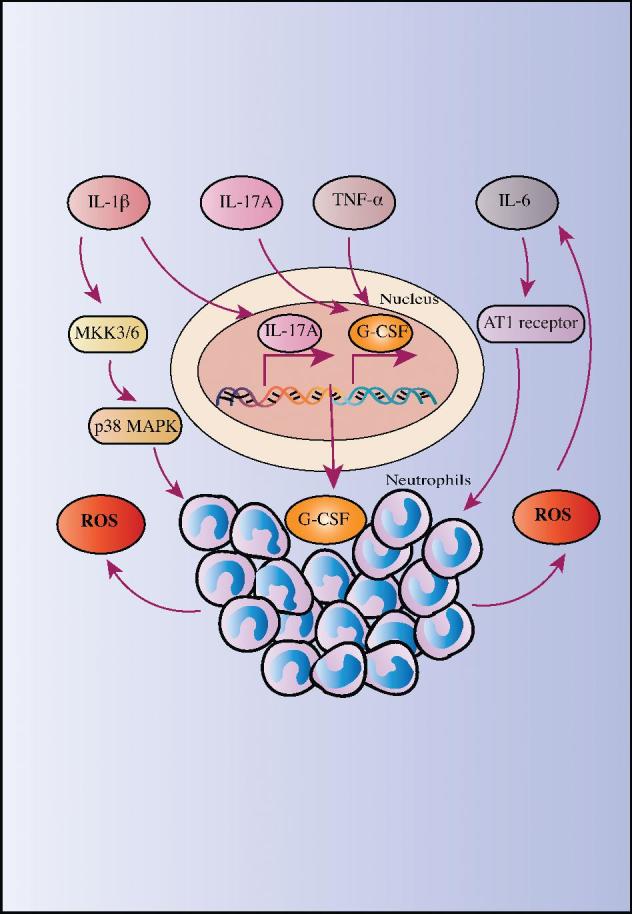
Inflammation provokes oxidative stress. Proinflammatory cytokines, such as IL-1β, IL-6, IL-17A, and TNF-α, can increase oxidative stress by recruiting neutrophils as well as inducing neutrophils to produce ROS. AT1 receptor, angiotensin II type 1 receptor; G-CSF, granulocyte colony-stimulating factor; MKK3/6, mitogen-activated protein kinase kinase 3/6; p38 MAPK, p38 mitogen-activated protein kinase; ROS, reactive oxygen species.
Chemotherapy- or Radiotherapy-Related Intestinal Microbial Dysbiosis and Deficiency of the Intestinal Barrier
The epithelial layer is known to be an important component of the mucosal barrier. In heathy individuals, tight junctions between epithelial cells play a pivotal role in maintaining the permeability of the intestinal epithelium, allowing for nutrient absorption while sequestering harmful substances to the lumen (69). In addition, the mucus layer covering the intestinal epithelium also contributes to mucosal barrier function. This layer consists of glycoproteins, mucins, immunoglobulins, and butyrate (34, 35, 70) (Figures 3 and 4). For example, mucin trimers build a biofilm that protects the epithelial cells from lumen toxins (78), and sIgA is a very important antibody able to neutralize toxins and pathogens in the mucus layer (70). In a healthy gut, some beneficial bacteria such as Lactobacillus and Streptococcus have been reported to promote the biosynthesis of sIgA, (79). Butyrate is able to promote mucin synthesis by upregulating the expression of the mucin 2 (MUC2) gene (80). In addition, butyrate is capable of promoting the secretion of cathelicidin, an antimicrobial peptide released by intestinal epithelial cells (81). Hence, butyrate-producing bacteria play a key role in maintaining the physiologic composition of mucus in a healthy gut. With these processes, the intestinal barrier is well maintained, thus improving the host's defense against lumen pathogens. However, when challenged by chemotherapy- or radiotherapy-related intestinal microbial dysbiosis, the permeability of the intestinal epithelium is increased and the mucus layer is interrupted to a certain extent due to intestinal microbial dysbiosis. In addition, the mucositis after chemotherapy or radiotherapy is always accompanied by impaired barrier function (Figure 3).
FIGURE 3.
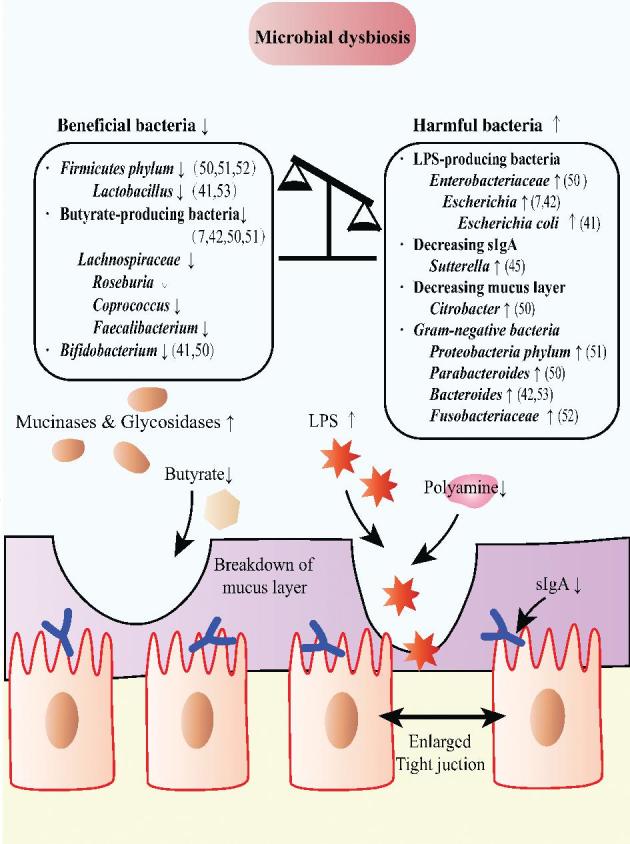
Chemotherapy- or radiotherapy-related intestinal microbial dysbiosis leads to dysfunction in the intestinal barrier. First, the intestinal barrier can be compromised by LPS-producing bacteria, leading to increased permeability. Second, the reduced proportion of butyrate-producing bacteria enables the mucus layer to be thinner than before. Third, gut concentrations of sIgA are decreased after chemotherapy or radiotherapy. sIgA, secretory IgA; ↑, increased; ↓, decreased.
FIGURE 4.
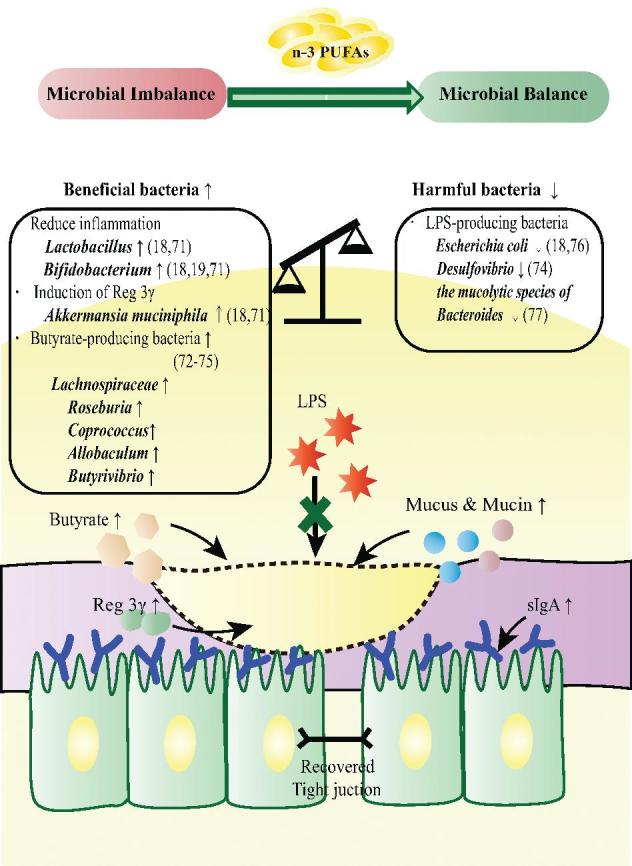
n–3 PUFAs attenuate chemotherapy- or radiotherapy-related intestinal microbial dysbiosis. n–3 PUFAs revert chemotherapy- or radiotherapy-related dysbiosis and maintain the intestinal barrier. Intake of n–3 PUFAs restores the beneficial microbiota via increasing the proportions of beneficial bacteria and reducing the proportions of harmful bacteria. As a result, the mucus layer is consolidated, intestinal permeability is reduced, and the concentration of sIgA is restored. Reg 3γ, regenerating islet derived protein 3γ; sIgA, secretory IgA; ↑, increased; ↓, decreased.
Chemotherapy- or radiotherapy-related intestinal microbial dysbiosis increases intestinal permeability
Intestinal microbial dysbiosis can increase intestinal permeability. For example, LPS has been shown to be capable of increasing intestinal permeability, which was mediated by an increased expression of TLR4 by enterocytes (Figure 3) (82). In addition to LPS, IL-1β could disrupt the tight junctions among epithelial cells by increasing the intracellular production of myosin light chain kinase (MLCK) (83). MLCK is capable of phosphorylating the myosin light chain (MLC) at serine residue 19 (84). The phosphorylated MLC subsequently activates Mg2+-myosin ATPase, resulting in the contraction among perijunctional actomyosin filaments and a widening of the intercellular spaces (83). However, if further challenged with chemotherapy, the epithelial permeability will deteriorate. For example, chemotherapy was reported to induce the loss of Clostridium XIVa in the gut (7). Clostridium XIVa physiologically maintains intestinal permeability by increasing the gut concentration of polyamine (85), a substance antagonizing LPS-induced intestinal dysfunction (86). In addition, both Bifidobacterium and Lactobacillus have been reported to be able to promote the expression of genes encoding tight junction proteins, such as occlaudin (87) and claudin (88). The intestinal proportions of such bacteria are always decreased after che-motherapy (41), enabling increases in epithelial permeability.
Chemotherapy- or radiotherapy-related intestinal microbial dysbiosis leads to breakdown of the mucus layer
Chemotherapy- or radiotherapy-related intestinal microbial dysbiosis could disrupt the mucus layer due to the decreased proportions of butyrate-producing bacteria (42, 50, 51), such as Roseburia, Coprococcus, and Faecalibacterium (89). In addition, the mucus layer could be directly destroyed by several bacteria; Citrobacter, which increases in the gut after chemotherapy (50), secretes mucinases and glycosidases, which corrode the mucus layer (90). Furthermore, chemotherapy increases the proportion of Enterobacteriaceae in the gut, which impairs the host's capacity to absorb cysteine, proline, and methionine from the diet (50), resulting in a reduction in the synthesis of mucin (91).
As mentioned above, sIgA is present in the mucus layer. However, the gut concentrations of sIgA may be decreased after radiotherapy because of intestinal microbial dysbiosis. For example, the increased proportion of Sutterella in the gut is a feature of radiation-related intestinal microbial dysbiosis (45). To investigate the relation between Sutterella and gut concentrations of sIgA, Moon et al. (92) observed that, when adding Sutterella into the culture system of intestinal epithelial cells in vitro, sIgA concentrations would be inadequate in the apical side of cells because Sutterella could degrade the bound secretory component, the cleaved form of the polymeric Ig receptor (pIgR). The bound secretory components on the intestinal epithelial cells assist in transporting the dimeric form of sIgA from the basolateral to the apical side of the epithelium and further prevent sIgA from being degraded by bacterial proteases (93). In an animal model, it was found that infection with Sutterella resulted in an sIgA-low phenotype, which could be inherited by the offspring (92). Moreover, the sIgA-low phenotype enabled the hosts to be predisposed to dextran sulfate sodium–induced colitis, suggesting the importance of Sutterella in decreasing gut sIgA concentrations (92).
Therapeutic Potential of n–3 PUFAs for Chemotherapy- or Radiotherapy-Related Intestinal Microbial Dysbiosis
Biosynthesis of n–3 PUFAs
The molecular structure of PUFAs contains no less than 18 carbon atoms, and there are ≥2 pairs of double bonds between the carbon atoms. According to the position of the first double bond, PUFAs can be divided into n–3 and n–6 FAs. Among these, linolenic acid (18:3n–3) and linoleic acid (18:2n–6) are the shortest n–3 and n–6 PUFAs, and they also serve as precursors of other n–3 and n–6 PUFAs (94). Linolenic acid can be converted into EPA (20:5n–3) (95), and after elongating and desaturating, EPA is finally converted into DHA (22:6n–3) using β-oxidation (95). EPA and DHA are referred to as the n–3 PUFAs (96). Linoleic acid, however, can be converted into eicosatetraenoic acid, which is an n–6 PUFA (97). Accumulating evidence has suggested that the n–3 PUFAs play a critical role in attenuating inflammation (98), whereas n–6 PUFAs are associated with proinflammatory responses due to their contribution to the biosynthesis of prostaglandin E2 (99). Moreover, n–3 PUFAs have been shown to exert antagonistic effects on intestinal microbial dysbiosis, resulting in an upregulated proportion of beneficial bacteria instead of harmful bacteria in the gut (Table 2). Therefore, n–3 PUFAs are candidates for managing chemotherapy- or radiotherapy-related intestinal microbial dysbiosis.
TABLE 2.
Clinical and preclinical studies associated with n–3 PUFAs affecting microbiota constitution1
| Study, year (ref) | Participants/n | Dietary supplement/duration | Techniques | Samples | Main findings |
|---|---|---|---|---|---|
| Watson et al., 2017 (19) | Healthy volunteers/20 | 4 g mixed DHA/EPA (1:1)/d for 8 wk | NGS | Feces | • Bacterial family: Clostridiaceae ↑, Sutterellaceae ↑, Akkermansiaceae ↑ |
| • Bacterial genus: Coprococcus ↓, Faecalibacterium ↓, Bifidobacterium ↑, Oscillospira ↑, Lachnospira ↑, Roseburia ↑ | |||||
| Menni et al., 2017 (73) | Female volunteers/876 | Estimated food intake of n–3 PUFAs were obtained from FFQs | NGS | Blood and feces | • 350 mg DNA/d led to a serumDHA concentration of 0.14 mmol/L• Microbiome α diversity ↑• Bacterial family: Lachnospiraceae family ↑, Ruminococcaceae family ↑ |
| Noriega et al., 2016 (72) | A healthy volunteer | 600 mg n–3 PUFAs (fish-protein diet)/d for 2 wk | NGS | Feces | • Bacterial phylum: Bacteroidetes ↓, Actinobacteria ↓, Firmicutes ↑• Bacterial genus: Faecalibacterium ↓, Blautia ↑, Roseburia ↑, Coprococcus ↑, Ruminococcus ↑, Subdoligranulum ↑ |
| Balfego et al., 2016 (100) | Patients with type 2 diabetes/35 | 3.0 g EPA and DHA from sardines for 5 d/wk for 6 mo | qPCR | Blood and feces | • Bacterial phylum: Firmicutes ↓, Firmicutes vs. Bacteroidetes ratio ↓• Bacterial genus: Bacteroides-Prevotella ↑ |
| Caesar et al., 2015 (71) | C57BL/6 mice | Experimental arm: diets enriched with menhaden fish oil for 11 wk; control arm: diets enriched with lard for 11 wk | 454 pyrosequencing | Feces | • Bacterial phylum: Actinobacteria ↑,Verrucomicrobia ↑• Bacterial class: Alphaproteobacteria ↑, Deltaproteobacteria ↑• Bacterial genus: Bifidobacterium ↑, Adlercreutzia ↑, Lactobacillus ↑, Streptococcus ↑• Bacterial species: Akkermansia muciniphila ↑ |
| Kaliannan et al., 2015 (18) | Fat1 +/− mice | Diet enriched with n–6 PUFAs (10% corn oil) or n–3 PUFAs (5% corn oil and 5% fish oil) for 8 mo | qPCR | Feces | • Intestinal tissue n–6:n–3 PUFA ratio ↓Low n-6:n-3 PUFA ratio led to:• LPS-suppressing and/or anti-inflammatory bacteria: Bifidobacterium ↑, Akkermansia muciniphila ↑, Clostridium clusters IV and XIVa ↑, Enterococcus faecium ↑, Lactobacillus gasseri ↑• LPS-producing and/or proinflammatory bacteria: Proteobacteria ↓, Enterobacteriaceae ↓, Escherichia coli ↓ gamma- and delta-proteobacteria ↓, Prevotella ↓, Fusobacterium ↓, Clostridium cluster XI ↓, segmented filamentous bacteria ↓ |
| Yu et al., 2014 (101) | Imprinting control region mice | Control arm: natural saline for 15 d; low-dose arm: fish oil (5 mg/kg) for 15 d; high-dose arm: fish oil (10 mg/kg) for 15 d (fish oil contained 40% EPA and 27% DHA) | PCR | Feces | • Bacterial genus: Helicobacter ↓, uncultured bacterium clone, WD2_aaf07d12 (GenBank: EU511712.1) ↓, Clostridiales bacterium ↓, Sphingomonadales bacterium ↓, Pseudomonas ↓, Firmicutes bacterium ↑ |
1 Fat1, FAT atypical cadherin 1; NGS, Next Generation Sequencing; ref, reference; ↓, decreased; ↑, increased.
n–3 PUFAs and increased beneficial bacteria
n–3 PUFAs can increase the gut proportions of beneficial bacteria (Figure 4). For example, Caesar et al. (71) found that mice fed fish-oil diets exhibited higher proportions of Lactobacillus and Akkermansia muciniphila in the gut than those fed lard. However, serum concentrations of LPS and bacterial DNA were obviously elevated along with high amounts of circulating proinflammatory cells after the lard intervention, reflecting the potential of fish oil to attenuate inflammation (71). To evaluate the anti-inflammatory effects of n–3 PUFAs rather than n–6 PUFAs in fish oil, Ghosh et al. (102) compared the severity of Citrobacter rodentium–induced colitis between mice fed diets with n–3 PUFAs or n–6 PUFAs for 5 wk. Relevant results showed that the mice fed the diets containing n–3 PUFAs exhibited more Lactobacillus and Bifidobacterium in the feces along with less gut inflammation than the mice fed diets containing n–6 PUFAs (102). Lactobacillus has been reported to be capable of suppressing the activation of the NF-κB signaling pathway by intracellularly stabilizing IκB (103), leading to downregulation of the expression of the genes encoding TNF-α and IL-8 and upregulation of IL10 expression within enterocytes (104). In addition, Lactobacillus could strengthen the phagotrophic function of macrophages (105), and Bifidobacterium was found to help maintain intestinal barrier function by inhibiting LPS-induced autophagy among enterocytes (106). In addition, Bifidobacterium could alleviate intestinal inflammation by decreasing IL-8 secretion from enterocytes (107) and increasing the amount of T-regulatory cells at injured sites (108). Moreover, treatment with n–3 PUFAs could increase the gut proportion of A. muciniphila (71), which induces Paneth cells to produce mucins and the antimicrobial peptide, the regenerating islet derived protein 3γ (Reg 3γ) (109).
As mentioned above, the proportions of butyrate-producing bacteria in the gut are commonly decreased after chemotherapy or radiotherapy (42, 50). Recent evidence suggests that the intake of n–3 PUFAs could increase the relative abundance of butyrate-producing bacteria in feces, such as Roseburia, Coprococcus, Allobaculum, and Butyrivibrio (72–75). In addition to its contributions to the formation of the mucus layer, butyrate also has anti-inflammatory functions (110). First, butyrate is able to inhibit NF-κB translocation into the nucleus by suppressing IκB degradation (111), resulting in high endogenous concentrations of IL-10 and low concentrations of IL-2 (112). Second, butyrate exclusively activates PPAR-γ, a nuclear receptor that antagonizes the expression of the gene encoding iNOS (113), and a decline in iNOS has been shown to hamper the production of nitrate by intestinal epithelial cells (114). Nitrate functions as a respiratory electron acceptor, which is essential for the reproduction of some pathogenic bacteria, such as Escherichia and Salmonella (114). When lacking nitrate, the gut proportions of these pathogenic bacteria decrease (77). Therefore, the high production of butyrate after an intervention with n–3 PUFAs can attenuate intestinal inflammation.
n–3 PUFAs and decreased harmful bacteria
As mentioned above, the typical feature of chemotherapy- or radiotherapy-related intestinal microbial dysbiosis is the increased gut proportions of harmful bacteria, such as LPS-producing bacteria and mucolytic bacteria (42, 50). Although E. coli and Desulfovibrio are capable of producing LPS (115), an animal study confirmed that Desulfovibrio infection also promoted the conversion of sulfates into sulfides (116), the latter of which corrupted the mucosal layer and resulted in ulcerative lesions (116). Likewise, the mucosal-adherent members of the Bacteroides include mucolytic species, and these mucolytic species can also impair the mucus layer (117). Recent evidence has confirmed that treatment with n–3 PUFAs could reduce the proportions of Desulfovibrio, E. coli, and the mucolytic species of Bacteroides (74, 76, 118) in the gut. Functionally, n–3 PUFAs have been reported to be capable of promoting the synthesis of intestinal alkaline phosphatase (IAP) (18), which could detoxify LPS through dephosphorylation (119). Moreover, n–3 PUFAs assisted in increasing the fluidity of the biomembrane by excluding cholesterol from the phospholipid layer (120), facilitating the bioactivity of IAP (121). Therefore, n–3 PUFAs can reduce the mucosal damage associated with intestinal microbial dysbiosis (Figure 4).
n–3 PUFAs and reduced inflammation
Intestinal microbial dysbiosis provokes inflammation within the gut after chemotherapy or radiotherapy. Nevertheless, recent studies have suggested that n–3 PUFAs could reduce the proinflammatory responses toward intestinal microbial dysbiosis (122). Functionally, n–3 PUFAs could directly block the signal transduction from TLR4 but not from its downstream molecules, such as MyD88 (Figure 5) (123). In this context, activation of the NF-κB signaling pathway was inhibited, thus resulting in decreased secretions of IL-1β, IL-6, and TNF-α (124–126). Apart from this action, DHA was shown to be capable of suppressing the bioactivity of the IκB kinase (IKK) complex by inhibiting its phosphorylation (Figure 5) (127). Meanwhile, IκB is a molecule that binds with NF-κB to block the translocation of NF-κB into the nucleus for subsequent activation of downstream gene expression (56). In this context, IκB is unable to be phosphorylated and is subsequently degraded by ubiquitination, resulting in the cytoplasmic accumulation of IκB (127). On this basis, transcriptional activation of NF-κB target genes was prohibited (56). Similarly, EPA and DHA could activate PPAR-γ (Figure 5) (128), which can antagonize the expression of NF-κB target genes by inhibiting the formation of the transcriptional complex of NF-κB (129), thus resulting in reduced inflammation.
FIGURE 5.
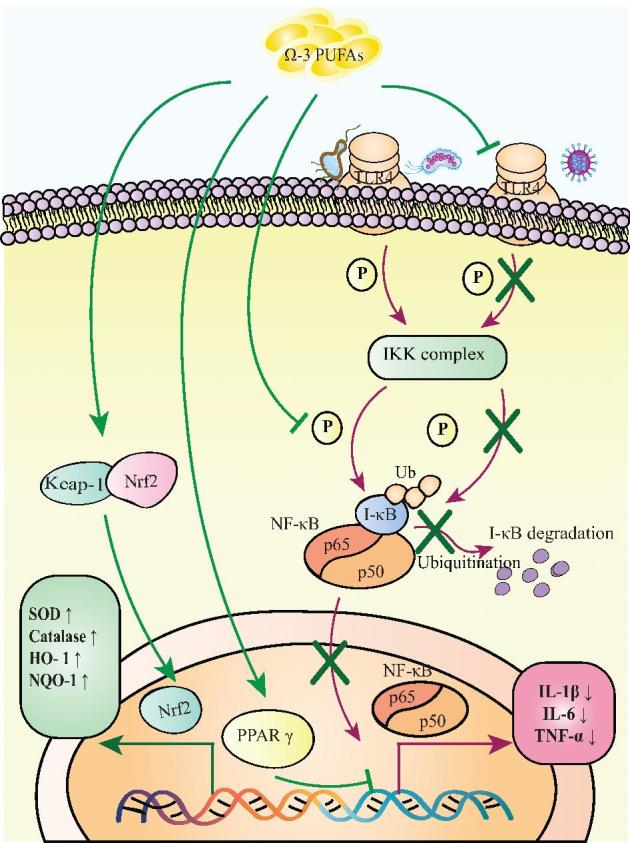
n–3 PUFAs attenuate inflammation and oxidative stress. Herein, n–3 PUFAs directly interact with TLR4, IKK, and PPAR-γ to inhibit the activation of NF-κB. As a result, secretions of IL-1β, IL-6, and TNF-α by stressed cells are inhibited. In addition, n–3 PUFAs can induce Nrf2 to dissociate from Keap1 to initiate the expressions of antioxidative genes encoding SOD, HO-1, and NQO-1. HO-1, heme-oxygenase 1; IKK, inhibitor of κB kinase; I-κB, inhibitor of κB; Keap1, Kelch-like ECH–associated protein 1; NQO-1, NAD(P)H-quinone oxidoreductase 1; Nrf2, nuclear factor erythroid 2 p45-related factor 2; P, phosphorylation; SOD, superoxide dismutase; TLR4, Toll-like receptor 4; Ub, ubiquitination; ↑, increase; ↓, decrease.
n–3 PUFAs and reduced oxidative stress
Intestinal inflammation and oxidative stress enable aggravation of tissue damage (130). Oxidative stress could induce the activation of the nuclear factor erythroid 2 p45–related factor 2 (Nrf2) signaling pathway to protect cells against oxidative damage (131). DHA was reported to be capable of switching on the Nrf2 signaling pathway to reduce oxidative stress, lessening the extent of tissue damage, because DHA and its derivative enabled Nrf2 to dissociate from Kelch-like ECH–associated protein 1 (Keap1) as a result of oxidative stress (132). Then, the free form of Nrf2 bound with the antioxidant response element within the nucleus, targeting the expression of genes encoding superoxide dismutase (SOD), catalase, heme-oxygenase 1, and NAD(P)H-quinone oxidoreductase 1 (133, 134). In the gut, different bacteria exhibited different tolerances to oxidative stress (135, 136), and the reduced extent of oxidative stress provided optimal conditions for the reproduction of beneficial bacteria (135). By contrast, oxidative stress favored the preservation of some harmful bacteria, such as E. coli and Enterococcus, because their OxyR proteins functioned as defenders against the oxidative burst by host macrophages after being engulfed (136). However, the antioxidant system in beneficial bacteria including most species of Lactobacillus was not as potent as the OxyR of E. coli in clearing ROS (135). Moreover, several species of Lactobacillus even lacked SOD (135). Therefore, using n–3 PUFAs to attenuate oxidative stress will allow for the maintenance of the proper proportions of some beneficial bacteria in the gut (Figure 5).
Anticancer Effect of n–3 PUFAs
Clinically, patients undergoing chemotherapy or radiotherapy always have solid tumors. Herein, the long-standing inflammation within a tumor bed potentially has negative impacts on tumor remission after chemotherapy or radiotherapy, partially due to the infiltration of some cancer-facilitating cells such as M2 macrophages and IL-17A–producing cells (137, 138). More recently, the evidence reported by Wong et al. (139) showed that oral administration of feces from patients with colorectal cancer (CRC) either to conventional mice or to germ-free mice promoted intestinal carcinogenesis due to the increased amount of Th17 cells within the gut tissue. Gut microbes can play a critical role in inflammation and other pathological processes. Nevertheless, the anti-inflammatory and antioxidative effects of n–3 PUFAs have been confirmed. Moreover, by crossing adenomatosis polyposis coli (Apc)min/+ mice with FAT atypical cadherin 1 (Fat1) mice, Han et al. (140) found that n–3 PUFAs could delay the formation of intestinal polyposis among the offspring. Likewise, previous basic studies also validated the anticancer effects of n–3 PUFAs by using immunodeficient mice bearing subcutaneous xenografts of HCT-116 and HT-29 cells (141, 142). Several clinical trials have been undertaken to evaluate the potential of n–3 PUFAs to reduce CRC risks in humans (Table 3).
TABLE 3.
Potential therapeutic uses of dietary n–3 PUFAs in patients with cancer1
| Study, year (ref) | Patients/n | Anticancer therapy | Dietary supplement/duration | Main findings |
|---|---|---|---|---|
| Ma et al., 2015 (143) | Gastric and colorectal cancer patients/99 | Surgery | Experimental arm: 80∼140 mg n–3 PUFAs/kg in intravenous fat emulsion/d for 8 d; control arm: soybean oil and medium-chain TGs in a lipid emulsion for 8 d | • Improved lipid metabolism: FFAs ↓, TGs ↓, HDL ↑• Attenuated inflammation: serum IL-6 ↓, serum C-reactive protein ↓, serum TNF-α ↓, serum procalcitonin ↓ |
| Faber et al., 2013 (144) | Cancer patients/38 | Radiotherapy | 3.6 g mixed DHA:EPA (1:2)/d for 7 d | • EPA and DHA in white blood cells ↑, serum PGE2 concentrations ↓ |
| Murff et al., 2012 (145) | Polyp-free control subjects/3166Adenomatous polyp patients/1597Hyperplastic polyp patients/544 | — | Dietary PUFA intake was calculated from FFQs | • Adequate intakes of n–3 PUFAs led to: production of PGE2 in women ↓, risk of colorectal adenomas in women ↓• Excessive intakes of α-linolenic acid led to: risk of hyperplastic polyps in men ↑ |
| West et al., 2010 (146) | Familial adenomatous polyposis/55 | Surgery | 2 g EPA-FFAs/d for 6 mo | • Polyp number ↓. sum of polyp diameters ↓, mucosal EPA concentrations ↑ |
1PGE2, prostaglandin E2; ref, reference; ↓, decreased; ↑, increased.
To show the anticancerous effect of n–3 PUFAs, the following mechanism is proposed. Activation of the Wnt/β-catenin signaling pathway drives the proliferation of CRC cells (147). n–3 PUFAs could distinctly reduce the synthesis of prostaglandin E2 (148), which has been reported to be capable of activating the Wnt/β-catenin signaling pathway by cross-talking with the protein kinase (PK) family members, including PKA, PKB, and PKC (149–151). With the addition of n–3 PUFAs, prostaglandin E2–induced proliferation among intestinal stem cells is controlled. In addition to reducing the synthesis of prostaglandin E2, EPA was reported to be the substrate of cyclo-oxygenase 2 (COX-2), which catalyzes EPA into prostaglandin E3 (152). Prostaglandin E3 acts as a counterpart of prostaglandin E2, thus limiting the proliferation among intestinal stem cells (153). In another study, the C57BL/6J mice bearing azoxymethane-dextran sulfate sodium–induced CRC exhibited high abundances of Lactobacillus in their gut after receiving EPA treatment, with accompanying reduced sizes of colorectal tumors, decreased amounts of proliferative cells, and increased amounts of apoptotic cells within the tumors (154). However, the mechanisms underlying the anticancerous effects of beneficial bacteria on CRC deserve further investigation.
n–3 PUFA Administration and Safety
According to recommendations from the Dietary Guidelines Advisory Committee in 2015, although no upper limit was given for dietary fat intake, SFAs should be replaced by PUFAs, suggesting the importance of PUFAs for human health. To date, the US FDA has approved several fish-oil health products. For preventing coronary artery disease, the recommended daily intake of fish oil for a heathy individual is 1 g, which contains ∼200–800 mg EPA + DHA (155). However, the most suitable dose for cancer patients remains to be determined, although daily doses of EPA + DHA ranging from 1.0 g to ∼7.0 g were found to be safe among patients with CRC (20). However, n–3 PUFAs are easily oxidized due to the presence of 6 double bonds, which makes these compounds susceptible to oxygen free radical attack (156). As a result, n–3 PUFAs are converted into malondialdehyde (MDA) and 4-oxo-2-nonenal (4-OHE) (156, 157). Thus, MDA is a biomarker reflecting the oxidation of n–3 PUFAs in vivo, and urinary MDA concentration is commonly tested after the intake of n–3 PUFAs (157). Recently, GC-MS was applied to quantify the concentrations of n–3 PUFAs in blood samples (158). In addition, DHA concentrations in erythrocytes or in plasma were found to predict organ DHA concentrations (159). Importantly, 4-OHE was determined to induce gene mutations by forming 4-OHE–DNA adducts (160). A recent study found that fecal extracts from rats fed n–3 PUFAs plus dietary oxidants exhibited higher intestinal toxicities than those supplemented with dietary oxidants alone (157). Hence, diet should be controlled in cancer patients receiving n–3 PUFAs. Red meat should be avoided because heme iron and myoglobin are able to oxidize n–3 PUFAs (157). Optimally, food containing high quantities of vitamin E, vitamin C, polyphenols, tocopherols, and carotenoids should be considered (161). In case of infection, fish oil should be avoided, because a previous study showed that fish oil could cause sepsis by impairing LPS dephosphorylation activity (102).
Conclusions
n–3 PUFAs could be capable of reverting chemotherapy- or radiotherapy-related intestinal microbial dysbiosis, attenuating intestinal inflammation and reducing oxidative stress in the gut. Therefore, administering n–3 PUFAs should be an option in these patients.
Acknowledgments
The authors’ responsibilities were as follows—PC and LD: wrote the review; YZ and BZ: edited the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by the National Natural Science Foundation of China (grants 81502751, 81773353 and 81874254), the Jilin Scientific and Technological Development Program (grant 20160520143JH), and the Norman Bethune Program of Jilin University (grant 2015319).
Author disclosures: YZ, BZ, LD, and PC, no conflicts of interest.
Abbreviations used: CRC, colorectal cancer; G-CSF, granulocyte colony-stimulating factor; iNOS, inducible NO synthase; IκB, inhibitor of κB; MDA, malondialdehyde; Nrf2, nuclear factor erythroid 2 p45–related factor 2; PK, protein kinase; ROS, reactive oxygen species; sIgA, secretory IgA; TLR, Toll-like receptor; 4-OHE, 4-oxo-2-nonenal.
References
- 1. Peterson DE, Bensadoun RJ, Roila F, Group EGW. Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann Oncol 2011;22(Suppl 6):vi78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qiu W, Carson-Walter EB, Liu H, Epperly M, Greenberger JS, Zambetti GP, Zhang L, Yu J. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell 2008;2(6):576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leibowitz BJ, Yang L, Wei L, Buchanan ME, Rachid M, Parise RA, Beumer JH, Eiseman JL, Schoen RE, Zhang L et al. . Targeting p53-dependent stem cell loss for intestinal chemoprotection. Sci Transl Med 2018;10 (427):eaam7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hauer-Jensen M, Denham JW, Andreyev HJ. Radiation enteropathy—pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol 2014;11(8):470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, McGuire DB, Hutchins RD, Peterson DE et al. . Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 2007;109(5):820–31. [DOI] [PubMed] [Google Scholar]
- 6. Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol 2014;16(7):1024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, Le Vacon F, de La Cochetiere MF. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—current evidence and potential clinical applications. Aliment Pharmacol Ther 2014;40(5):409–21. [DOI] [PubMed] [Google Scholar]
- 8. Khokhlova EV, Smeianov VV, Efimov BA, Kafarskaia LI, Pavlova SI, Shkoporov AN. Anti-inflammatory properties of intestinal Bifidobacterium strains isolated from healthy infants. Microbiol Immunol 2012;56(1):27–39. [DOI] [PubMed] [Google Scholar]
- 9. van Vliet MJ, Harmsen HJ, de Bont ES, Tissing WJ. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog 2010;6(5):e1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ, Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science 2012;336(6086):1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hiscott J, Marois J, Garoufalis J, D'Addario M, Roulston A, Kwan I, Pepin N, Lacoste J, Nguyen H, Bensi G et al. . Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol Cell Biol 1993;13(10):6231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med 1990;171(1):35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Son YH, Jeong YT, Lee KA, Choi KH, Kim SM, Rhim BY, Kim K. Roles of MAPK and NF-kappaB in interleukin-6 induction by lipopolysaccharide in vascular smooth muscle cells. J Cardiovasc Pharmacol 2008;51(1):71–7. [DOI] [PubMed] [Google Scholar]
- 14. Manzi L, Costantini L, Molinari R, Merendino N. Effect of dietary omega-3 polyunsaturated fatty acid DHA on glycolytic enzymes and Warb urg phenotypes in cancer. Biomed Res Int 2015;2015:137097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calder PC. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res 2008;52(8):885–97. [DOI] [PubMed] [Google Scholar]
- 16. Zhao J, Shi P, Sun Y, Sun J, Dong JN, Wang HG, Zuo LG, Gong JF, Li Y, Gu LL et al. . DHA protects against experimental colitis in IL-10-deficient mice associated with the modulation of intestinal epithelial barrier function. Br J Nutr 2015;114(2):181–8. [DOI] [PubMed] [Google Scholar]
- 17. Lam CF, Katusic ZS. Genetic modification of vascular endothelial function as therapeutic strategy in heart failure. Am J Physiol Heart Circ Physiol 2005;289(2):H518–9. [DOI] [PubMed] [Google Scholar]
- 18. Kaliannan K, Wang B, Li XY, Kim KJ, Kang JX. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci Rep 2015;5:11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watson H, Mitra S, Croden FC, Taylor M, Wood HM, Perry SL, Spencer JA, Quirke P, Toogood GJ, Lawton CL et al. . A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2017;0:1–10. [DOI] [PubMed] [Google Scholar]
- 20. Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut 2012;61(1):135–49. [DOI] [PubMed] [Google Scholar]
- 21. Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev 2010;90(3):859–904. [DOI] [PubMed] [Google Scholar]
- 22. Thomas F, Hehemann JH, Rebuffet E, Czjzek M, Michel G. Environmental and gut Bacteroidetes: the food connection. Front Microbiol 2011;2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science 2005;308(5728):1635–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature 2012;486(7402):207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maier E, Anderson RC, Roy NC. Understanding how commensal obligate anaerobic bacteria regulate immune functions in the large intestine. Nutrients 2015;7(1):45–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heinken A, Thiele I. Anoxic conditions promote species-specific mutualism between gut microbes in silico. Appl Environ Microbiol 2015;81(12):4049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evaldson G, Heimdahl A, Kager L, Nord CE. The normal human anaerobic microflora. Scand J Infect Dis Suppl 1982;35:9–15. [PubMed] [Google Scholar]
- 28. Sundin OH, Mendoza-Ladd A, Zeng M, Diaz-Arevalo D, Morales E, Fagan BM, Ordonez J, Velez P, Antony N, McCallum RW. The human jejunum has an endogenous microbiota that differs from those in the oral cavity and colon. BMC Microbiol 2017;17(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin L, Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol 2017;18(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol 2016;16(6):341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. LeBlanc JG, Laino JE, del Valle MJ, Vannini V, van Sinderen D, Taranto MP, de Valdez GF, de Giori GS, Sesma F. B-group vitamin production by lactic acid bacteria—current knowledge and potential applications. J Appl Microbiol 2011;111(6):1297–309. [DOI] [PubMed] [Google Scholar]
- 32. Conly JM, Stein K. The production of menaquinones (vitamin K2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Prog Food Nutr Sci 1992;16(4):307–43. [PubMed] [Google Scholar]
- 33. Martin R, Miquel S, Ulmer J, Langella P, Bermudez-Humaran LG. Gut ecosystem: how microbes help us. Benef Microbes 2014;5(3):219–33. [DOI] [PubMed] [Google Scholar]
- 34. Puertollano E, Kolida S, Yaqoob P. Biological significance of short-chain fatty acid metabolism by the intestinal microbiome. Curr Opin Clin Nutr Metab Care 2014;17(2):139–44. [DOI] [PubMed] [Google Scholar]
- 35. Cornick S, Tawiah A, Chadee K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers 2015;3(1–2):e982426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gerard P. Metabolism of cholesterol and bile acids by the gut microbiota. Pathogens 2013;3(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duda-Chodak A, Tarko T, Satora P, Sroka P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur J Nutr 2015;54(3):325–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martin R, Miquel S, Ulmer J, Langella P, Bermudez-Humaran LG. Gut ecosystem: how microbes help us. Benef Microbes 2014;5(3):219–33. [DOI] [PubMed] [Google Scholar]
- 39. Kolling GL, Wu M, Warren CA, Durmaz E, Klaenhammer TR, Timko MP, Guerrant RL. Lactic acid production by Streptococcus thermophilus alters Clostridium difficile infection and in vitro toxin A production. Gut Microbes 2012;3(6):523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol 2006;290(4):C1018–30. [DOI] [PubMed] [Google Scholar]
- 41. Stringer AM, Al-Dasooqi N, Bowen JM, Tan TH, Radzuan M, Logan RM, Mayo B, Keefe DM, Gibson RJ. Biomarkers of chemotherapy-induced diarrhea: a clinical study of intestinal microbiome alterations, inflammation and circulating matrix metalloproteinases. Support Care Cancer 2013;21(7):1843–52. [DOI] [PubMed] [Google Scholar]
- 42. Wang A, Ling Z, Yang Z, Kiela PR, Wang T, Wang C, Cao L, Geng F, Shen M, Ran X et al. . Gut microbial dysbiosis may predict diarrhea and fatigue in patients undergoing pelvic cancer radiotherapy: a pilot study. PLoS One 2015;10(5):e0126312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chang PY, Qu YQ, Wang J, Dong LH. The potential of mesenchymal stem cells in the management of radiation enteropathy. Cell Death Dis 2015;6:e1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Vliet MJ, Harmsen HJM, de Bont ESJM, Tissing WJE. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PloS Pathog 2010;6(5):e1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gerassy-Vainberg S, Blatt A, Danin-Poleg Y, Gershovich K, Sabo E, Nevelsky A, Daniel S, Dahan A, Ziv O, Dheer R et al. . Radiation induces proinflammatory dysbiosis: transmission of inflammatory susceptibility by host cytokine induction. Gut 2018;67(1):97–107. [DOI] [PubMed] [Google Scholar]
- 46. Crawford PA, Gordon JI. Microbial regulation of intestinal radiosensitivity. Proc Natl Acad Sci USA 2005;102(37):13254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ferreira MR, Muls A, Dearnaley DP, Andreyev HJ. Microbiota and radiation-induced bowel toxicity: lessons from inflammatory bowel disease for the radiation oncologist. Lancet Oncol 2014;15(3):e139–47. [DOI] [PubMed] [Google Scholar]
- 48. Olaya J, Neopikhanov V, Uribe A. Lipopolysaccharide of Escherichia coli, polyamines, and acetic acid stimulate cell proliferation in intestinal epithelial cells. In Vitro Cell Dev Biol Anim 1999;35(1):43–8. [DOI] [PubMed] [Google Scholar]
- 49. Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, Gao R, Liu M, Yin M, Pan C et al. . Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating Toll-like receptor 4 signaling to nuclear factor-kappaB, and up-regulating expression of microRNA-21. Gastroenterology 2017;152(4):851–66, e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Montassier E, Gastinne T, Vangay P, Al-Ghalith GA, des Varannes SB, Massart S, Moreau P, Potel G, de La Cochetiere MF, Batard E et al. . Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharm Ther 2015;42(5):515–28. [DOI] [PubMed] [Google Scholar]
- 51. Montassier E, Batard E, Massart S, Gastinne T, Carton T, Caillon J, Le Fresne S, Caroff N, Hardouin JB, Moreau P et al. . 16S rRNA gene pyrosequencing reveals shift in patient faecal microbiota during high-dose chemotherapy as conditioning regimen for bone marrow transplantation. Microb Ecol 2014;67(3):690–9. [DOI] [PubMed] [Google Scholar]
- 52. Nam YD, Kim HJ, Seo JG, Kang SW, Bae JW. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing. PLoS One 2013;8(12):e82659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zwielehner J, Lassl C, Hippe B, Pointner A, Switzeny OJ, Remely M, Kitzweger E, Ruckser R, Haslberger AG. Changes in human fecal microbiota due to chemotherapy analyzed by TaqMan-PCR, 454 sequencing and PCR-DGGE fingerprinting. PLoS One 2011;6(12):e28654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Manichanh C, Varela E, Martinez C, Antolin M, Llopis M, Dore J, Giralt J, Guarner F, Malagelada JR. The gut microbiota predispose to the pathophysiology of acute postradiotherapy diarrhea. Am J Gastroenterol 2008;103(7):1754–61. [DOI] [PubMed] [Google Scholar]
- 55. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006;124(4):783–801. [DOI] [PubMed] [Google Scholar]
- 56. Wullaert A. Role of NF-kappaB activation in intestinal immune homeostasis. Int J Med Microbiol 2010;300(1):49–56. [DOI] [PubMed] [Google Scholar]
- 57. Sonis ST. The pathobiology of mucositis. Nat Rev Cancer 2004;4(4):277–84. [DOI] [PubMed] [Google Scholar]
- 58. Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G et al. . Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA 2008;105(43):16731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van Vliet MJ, Tissing WJ, Dun CA, Meessen NE, Kamps WA, de Bont ES, Harmsen HJ. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin Infect Dis 2009;49(2):262–70. [DOI] [PubMed] [Google Scholar]
- 60. Lakhdari O, Tap J, Beguet-Crespel F, Le Roux K, de Wouters T, Cultrone A, Nepelska M, Lefevre F, Dore J, Blottiere HM. Identification of NF-kappaB modulation capabilities within human intestinal commensal bacteria. J Biomed Bi otechnol 2011;2011:282356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Suzuki K, Hino M, Kutsuna H, Hato F, Sakamoto C, Takahashi T, Tatsumi N, Kitagawa S. Selective activation of p38 mitogen-activated protein kinase cascade in human neutrophils stimulated by IL-1beta. J Immunol 2001;167(10):5940–7. [DOI] [PubMed] [Google Scholar]
- 62. Coccia M, Harrison OJ, Schiering C, Asquith MJ, Becher B, Powrie F, Maloy KJ. IL-1beta mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med 2012;209(9):1595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol 2012;181(1):8–18. [DOI] [PubMed] [Google Scholar]
- 64. Koeffler HP, Gasson J, Ranyard J, Souza L, Shepard M, Munker R. Recombinant human TNF alpha stimulates production of granulocyte colony-stimulating factor. Blood 1987;70(1):55–9. [PubMed] [Google Scholar]
- 65. Wang J, Arase H. Regulation of immune responses by neutrophils. Ann NY Acad Sci 2014;1319:66–81. [DOI] [PubMed] [Google Scholar]
- 66. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 2009;27:519–50. [DOI] [PubMed] [Google Scholar]
- 67. Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Bohm M, Nickenig G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res 2004;94(4):534–41. [DOI] [PubMed] [Google Scholar]
- 68. Volonte D, Zou H, Bartholomew JN, Liu Z, Morel PA, Galbiati F. Oxidative stress-induced inhibition of Sirt1 by caveolin-1 promotes p53-dependent premature senescence and stimulates the secretion of interleukin 6 (IL-6). J Biol Chem 2015;290(7):4202–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol 2003;18(5):479–97. [DOI] [PubMed] [Google Scholar]
- 70. Mantis NJ, Rol N, Corthesy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol 2011;4(6):603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Backhed F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab 2015;22(4):658–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Noriega BS, Sanchez-Gonzalez MA, Salyakina D, Coffman J. Understanding the impact of omega-3 rich diet on the gut microbiota. Case Rep Med 2016;2016:3089303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Menni C, Zierer J, Pallister T, Jackson MA, Long T, Mohney RP, Steves CJ, Spector TD, Valdes AM. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci Rep 2017;7(1):11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wan J, Hu S, Jacoby JJ, Liu J, Zhang Y, Yu LL. The impact of dietary sn-2 palmitic triacylglycerols in combination with docosahexaenoic acid or arachidonic acid on lipid metabolism and host faecal microbiota composition in Sprague Dawley rats. Food Funct 2017;8(5):1793–802. [DOI] [PubMed] [Google Scholar]
- 75. Pusceddu MM, El Aidy S, Crispie F, O'Sullivan O, Cotter P, Stanton C, Kelly P, Cryan JF, Dinan TG n-3 Polyunsaturated fatty acids (PUFAs) reverse the impact of early-life stress on the gut microbiota. PLoS One 2015;10(10):e0139721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yin J, Lee KY, Kim JK, Kim IH. Effects of different n-6 to n-3 polyunsaturated fatty acids ratio on reproductive performance, fecal microbiota and nutrient digestibility of gestation-lactating sows and suckling piglets. Anim Sci J 2017;88(11):1744–52. [DOI] [PubMed] [Google Scholar]
- 77. Byndloss MX, Olsan EE, Rivera-Chavez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y et al. . Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 2017;357(6351):570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 2010;12(5):319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Perdigon G, Vintini E, Alvarez S, Medina M, Medici M. Study of the possible mechanisms involved in the mucosal immune system activation by lactic acid bacteria. J Dairy Sci 1999;82(6):1108–14. [DOI] [PubMed] [Google Scholar]
- 80. Burger-van Paassen N, Vincent A, Puiman PJ, van der Sluis M, Bouma J, Boehm G, van Goudoever JB, Van Seuningen I, Renes IB. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J 2009;420:211–9. [DOI] [PubMed] [Google Scholar]
- 81. Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun 2002;70(2):953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol 2013;182(2):375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Al-Sadi R, Ye DM, Said HM, Ma TY. IL-1 beta-induced increase in intestinal epithelial tight junction permeability is mediated by MEKK-1 activation of canonical NF-kappa B pathway. Am J Pathol 2010;177(5):2310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shen Q, Rigor RR, Pivetti CD, Wu MH, Yuan SY. Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc Res 2010;87(2):272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Matsumoto M, Benno Y. The relationship between microbiota and polyamine concentration in the human intestine: a pilot study. Microbiol Immunol 2007;51(1):25–35. [DOI] [PubMed] [Google Scholar]
- 86. Chen J, Rao JN, Zou T, Liu L, Marasa BS, Xiao L, Zeng X, Turner DJ, Wang JY. Polyamines are required for expression of Toll-like receptor 2 modulating intestinal epithelial barrier integrity. Am J Physiol Gastrointest Liver Physiol 2007;293(3):G568–76. [DOI] [PubMed] [Google Scholar]
- 87. Hsieh CY, Osaka T, Moriyama E, Date Y, Kikuchi J, Tsuneda S. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol Rep 2015;3 (3):e12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sultana R, McBain AJ, O'Neill CA. Strain-dependent augmentation of tight-junction barrier function in human primary epidermal keratinocytes by Lactobacillus and Bifidobacterium lysates. Appl Environ Microbiol 2013;79(16):4887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol 2017;19(1):29–41. [DOI] [PubMed] [Google Scholar]
- 90. Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB et al. . Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog 2010;6(5):e1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Faure M, Mettraux C, Moennoz D, Godin JP, Vuichoud J, Rochat F, Breuille D, Obled C, Corthesy-Theulaz I. Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium-treated rats. J Nutr 2006;136(6):1558–64. [DOI] [PubMed] [Google Scholar]
- 92. Moon C, Baldridge MT, Wallace MA, Carey-Ann D, Burnham, Virgin HW, Stappenbeck TS. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature 2015;521(7550):90–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lindh E. Increased resistance of immunoglobulin A dimers to proteolytic degradation after binding of secretory component. J Immunol 1975;114(1 Part 2):284–6. [PubMed] [Google Scholar]
- 94. Miccadei S, Masella R, Mileo AM, Gessani S. omega3 Polyunsaturated fatty acids as immunomodulators in colorectal cancer: new potential role in adjuvant therapies. Front Immunol 2016;7:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gleissman H, Johnsen JI, Kogner P. Omega-3 fatty acids in cancer, the protectors of good and the killers of evil? Exp Cell Res 2010;316(8):1365–73. [DOI] [PubMed] [Google Scholar]
- 96. Burdge G. Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr Opin Clin Nutr Metab Care 2004;7(2):137–44. [DOI] [PubMed] [Google Scholar]
- 97. Burdge GC, Calder PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev 2005;45(5):581–97. [DOI] [PubMed] [Google Scholar]
- 98. Wang W, Zhu J, Lyu F, Panigrahy D, Ferrara KW, Hammock B, Zhang G. omega-3 Polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins Other Lipid Mediat 2014;113-115:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci USA 2003;100(4):1751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Balfego M, Canivell S, Hanzu FA, Sala-Vila A, Martinez-Medina M, Murillo S, Mur T, Ruano EG, Linares F, Porras N et al. . Effects of sardine-enriched diet on metabolic control, inflammation and gut microbiota in drug-naive patients with type 2 diabetes: a pilot randomized trial. Lipids Health Dis 2016;15:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yu HN, Zhu J, Pan WS, Shen SR, Shan WG, Das UN. Effects of fish oil with a high content of n-3 polyunsaturated fatty acids on mouse gut microbiota. Arch Med Res 2014;45(3):195–202. [DOI] [PubMed] [Google Scholar]
- 102. Ghosh S, DeCoffe D, Brown K, Rajendiran E, Estaki M, Dai C, Yip A, Gibson DL. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS One 2013;8(2):e55468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tien MT, Girardin SE, Regnault B, Le Bourhis L, Dillies MA, Coppee JY, Bourdet-Sicard R, Sansonetti PJ, Pedron T. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J Immunol 2006;176(2):1228–37. [DOI] [PubMed] [Google Scholar]
- 104. Borruel N, Casellas F, Antolin M, Llopis M, Carol M, Espiin E, Naval J, Guarner F, Malagelada JR. Effects of nonpathogenic bacteria on cytokine secretion by human intestinal mucosa. Am J Gastroenterol 2003;98(4):865–70. [DOI] [PubMed] [Google Scholar]
- 105. Sun L, Liu H, Jiang H, Wei M, Liang S, Wang M, Shi K, He Q. Macrophages are involved in gut bacterial translocation and reversed by lactobacillus in experimental uremia. Dig Dis Sci 2016;61(6):1534–44. [DOI] [PubMed] [Google Scholar]
- 106. Han C, Ding Z, Shi H, Qian W, Hou X, Lin R. The role of probiotics in lipopolysaccharide-induced autophagy in intestinal epithelial cells. Cell Physiol Biochem 2016;38(6):2464–78. [DOI] [PubMed] [Google Scholar]
- 107. Bai AP, Ouyang Q, Zhang W, Wang CH, Li SF. Probiotics inhibit TNF-alpha-induced interleukin-8 secretion of HT29 cells. World J Gastroenterol 2004;10(3):455–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. O'Mahony C, Scully P, O'Mahony D, Murphy S, O'Brien F, Lyons A, Sherlock G, MacSharry J, Kiely B, Shanahan F et al. . Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog 2008;4(8):e1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hanninen A, Toivonen R, Poysti S, Belzer C, Plovier H, Ouwerkerk JP, Emani R, Cani PD, De Vos WM. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut 2018;67(8):1445–53. [DOI] [PubMed] [Google Scholar]
- 110. Liu H, Wang J, He T, Becker S, Zhang G, Li D, Ma X. Butyrate: a double-edged sword for health? Adv Nutr 2018;9(1):21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yin L, Laevsky G, Giardina C. Butyrate suppression of colonocyte NF-kappa B activation and cellular proteasome activity. J Biol Chem 2001;276(48):44641–6. [DOI] [PubMed] [Google Scholar]
- 112. Saemann MD, Bohmig GA, Osterreicher CH, Burtscher H, Parolini O, Diakos C, Stockl J, Horl WH, Zlabinger GJ. Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J 2000;14(15):2380–2. [DOI] [PubMed] [Google Scholar]
- 113. Russo R, De Caro C, Avagliano C, Cristiano C, La Rana G, Mattace Raso G, Berni Canani R, Meli R, Calignano A. Sodium butyrate and its synthetic amide derivative modulate nociceptive behaviors in mice. Pharmacol Res 2016;103:279–91. [DOI] [PubMed] [Google Scholar]
- 114. Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD et al. . Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 2013;339(6120):708–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wolny D, Lodowska J, Jaworska-Kik M, Kurkiewicz S, Weglarz L, Dzierzewicz Z. Chemical composition of Desulfovibrio desulfuricans lipid A. Arch Microbiol 2011;193(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rowan FE, Docherty NG, Coffey JC, O'Connell PR. Sulphate-reducing bacteria and hydrogen sulphide in the aetiology of ulcerative colitis. Br J Surg 2009;96(2):151–8. [DOI] [PubMed] [Google Scholar]
- 117. Ouwerkerk JP, de Vos WM, Belzer C. Glycobiome: bacteria and mucus at the epithelial interface. Best Pract Res Clin Gastroenterol 2013;27(1):25–38. [DOI] [PubMed] [Google Scholar]
- 118. Prossomariti A, Scaioli E, Piazzi G, Fazio C, Bellanova M, Biagi E, Candela M, Brigidi P, Consolandi C, Balbi T et al. . Short-term treatment with eicosapentaenoic acid improves inflammation and affects colonic differentiation markers and microbiota in patients with ulcerative colitis. Sci Rep 2017;7(1):7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lalles JP. Intestinal alkaline phosphatase: novel functions and protective effects. Nutr Rev 2014;72(2):82–94. [DOI] [PubMed] [Google Scholar]
- 120. Chapkin RS, McMurray DN, Davidson LA, Patil BS, Fan YY, Lupton JR. Bioactive dietary long-chain fatty acids: emerging mechanisms of action. Br J Nutr 2008;100(6):1152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sharma U, Singh SK, Pal D, Khajuria R, Mandal AK, Prasad R. Implication of BBM lipid composition and fluidity in mitigated alkaline phosphatase activity in renal cell carcinoma. Mol Cell Biochem 2012;369(1-2):287–93. [DOI] [PubMed] [Google Scholar]
- 122. Costantini L, Molinari R, Farinon B, Merendino N. Impact of omega-3 fatty acids on the gut microbiota. Int J Mol Sci 2017;18(12): E2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res 2003;44(3):479–86. [DOI] [PubMed] [Google Scholar]
- 124. Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab 2006;91(2):439–46. [DOI] [PubMed] [Google Scholar]
- 125. Liang B, Wang S, Ye YJ, Yang XD, Wang YL, Qu J, Xie QW, Yin MJ. Impact of postoperative omega-3 fatty acid-supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World J Gastroenterol 2008;14(15):2434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Davidson J, Higgs W, Rotondo D. Eicosapentaenoic acid suppression of systemic inflammatory responses and inverse up-regulation of 15-deoxyDelta(12,14) prostaglandin J2 production. Br J Pharmacol 2013;169(5):1130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan WQ, Li PP, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010;142(5):687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Zapata-Gonzalez F, Rueda F, Petriz J, Domingo P, Villarroya F, Diaz-Delfin J, de Madariaga MA, Domingo JC. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through PPAR gamma: RXR heterodimers: comparison with other polyunsaturated fatty acids. J Leukocyte Biol 2008;84(4):1172–82. [DOI] [PubMed] [Google Scholar]
- 129. De Bosscher K, Vanden Berghe W, Haegeman G. Cross-talk between nuclear receptors and nuclear factor kappa B. Oncogene 2006;25(51):6868–86. [DOI] [PubMed] [Google Scholar]
- 130. Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 2014;94(2):329–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Yamadori T, Ishii Y, Homma S, Morishima Y, Kurishima K, Itoh K, Yamamoto M, Minami Y, Noguchi M, Hizawa N. Molecular mechanisms for the regulation of Nrf2-mediated cell proliferation in non-small-cell lung cancers. Oncogene 2012;31(45):4768–77. [DOI] [PubMed] [Google Scholar]
- 132. Ishikado A, Morino K, Nishio Y, Nakagawa F, Mukose A, Sono Y, Yoshioka N, Kondo K, Sekine O, Yoshizaki T et al. . 4-Hydroxy hexenal derived from docosahexaenoic acid protects endothelial cells via Nrf2 activation. PLoS One 2013;8(7):e69415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med 2004;36(10):1199–207. [DOI] [PubMed] [Google Scholar]
- 134. Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett 2005;579(14):3029–36. [DOI] [PubMed] [Google Scholar]
- 135. Roy DG, Klaenhammer TR, Hassan HM. Cloning and expression of the manganese superoxide dismutase gene of Escherichia coli in Lactococcus lactis and Lactobacillus gasseri. Mol Gen Genet 1993;239(1–2):33–40. [DOI] [PubMed] [Google Scholar]
- 136. Qiao Y, Sun J, Ding YY, Le GW, Shi YH. Alterations of the gut microbiota in high-fat diet mice is strongly linked to oxidative stress. Appl Microbiol Biot 2013;97(4):1689–97. [DOI] [PubMed] [Google Scholar]
- 137. De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 2013;23(3):277–86. [DOI] [PubMed] [Google Scholar]
- 138. Patil RS, Bhat SA, Dar AA, Chiplunkar SV. The Jekyll and Hyde story of IL17-producing gammadeltaT cells. Front Immunol 2015;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, Xiao X, Kwong TNY, Tsoi H, Wu WKK et al. . Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology 2017;153(6):1621–33e6. [DOI] [PubMed] [Google Scholar]
- 140. Han YM, Park JM, Cha JY, Jeong M, Go EJ, Hahm KB. Endogenous conversion of-6 to-3 polyunsaturated fatty acids in Fat-1 mice attenuated intestinal polyposis by either inhibiting COX-2/-catenin signaling or activating 15-PGDH/IL-18. Int J Cancer 2016;138(9):2247–56. [DOI] [PubMed] [Google Scholar]
- 141. Boudreau MD, Sohn KH, Rhee SH, Lee SW, Hunt JD, Hwang DH. Suppression of tumor cell growth both in nude mice and in culture by n-3 polyunsaturated fatty acids: mediation through cyclooxygenase-independent pathways. Cancer Res 2001;61(4):1386–91. [PubMed] [Google Scholar]
- 142. Calviello G, Di Nicuolo F, Gragnoli S, Piccioni E, Serini S, Maggiano N, Tringali G, Navarra P, Ranelletti FO, Palozza P. n-3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-1 and -2 and HIF-1alpha induction pathway. Carcinogenesis 2004;25(12):2303–10. [DOI] [PubMed] [Google Scholar]
- 143. Ma CJ, Wu JM, Tsai HL, Huang CW, Lu CY, Sun LC, Shih YL, Chen CW, Chuang JF, Wu MH et al. . Prospective double-blind randomized study on the efficacy and safety of an n-3 fatty acid enriched intravenous fat emulsion in postsurgical gastric and colorectal cancer patients. Nutr J 2015;14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Faber J, Berkhout M, Fiedler U, Avlar M, Witteman BJ, Vos AP, Henke M, Garssen J, van Helvoort A, Otten MH et al. . Rapid EPA and DHA incorporation and reduced PGE2 levels after one week intervention with a medical food in cancer patients receiving radiotherapy, a randomized trial. Clin Nutr 2013;32(3):338–45. [DOI] [PubMed] [Google Scholar]
- 145. Murff HJ, Shrubsole MJ, Cai Q, Smalley WE, Dai Q, Milne GL, Ness RM, Zheng W. Dietary intake of PUFAs and colorectal polyp risk. Am J Clin Nutr 2012;95(3):703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. West NJ, Clark SK, Phillips RK, Hutchinson JM, Leicester RJ, Belluzzi A, Hull MA. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut 2010;59(7):918–25. [DOI] [PubMed] [Google Scholar]
- 147. Cui S, Chang PY. Current understanding concerning intestinal stem cells. World J Gastroenterol 2016;22(31):7099–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Vanamala J, Glagolenko A, Yang P, Carroll RJ, Murphy ME, Newman RA, Ford JR, Braby LA, Chapkin RS, Turner ND et al. . Dietary fish oil and pectin enhance colonocyte apoptosis in part through suppression of PPARdelta/PGE2 and elevation of PGE3. Carcinogenesis 2008;29(4):790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT et al. . Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 2009;136(6):1136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science 2005;310(5753):1504–10. [DOI] [PubMed] [Google Scholar]
- 151. Lima JB, Araujo-Santos T, Lazaro-Souza M, Carneiro AB, Ibraim IC, Jesus-Santos FH, Luz NF, Pontes SM, Entringer PF, Descoteaux A et al. . Leishmania infantum lipophosphoglycan induced-Prostaglandin E2 production in association with PPAR-gamma expression via activation of Toll like receptors-1 and 2. Sci Rep 2017;7(1):14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Yang P, Chan D, Felix E, Cartwright C, Menter DG, Madden T, Klein RD, Fischer SM, Newman RA. Formation and antiproliferative effect of prostaglandin E(3) from eicosapentaenoic acid in human lung cancer cells. J Lipid Res 2004;45(6):1030–9. [DOI] [PubMed] [Google Scholar]
- 153. Fan YY, Davidson LA, Callaway ES, Goldsby JS, Chapkin RS. Differential effects of 2- and 3-series E-prostaglandins on in vitro expansion of Lgr5+ colonic stem cells. Carcinogenesis 2014;35(3):606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Piazzi G, D'Argenio G, Prossomariti A, Lembo V, Mazzone G, Candela M, Biagi E, Brigidi P, Vitaglione P, Fogliano V et al. . Eicosapentaenoic acid free fatty acid prevents and suppresses colonic neoplasia in colitis-associated colorectal cancer acting on Notch signaling and gut microbiota. Int J Cancer 2014;135(9):2004–13. [DOI] [PubMed] [Google Scholar]
- 155. Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 2006;296(15):1885–99. [DOI] [PubMed] [Google Scholar]
- 156. Yum HW, Na HK, Surh YJ. Anti-inflammatory effects of docosahexaenoic acid: implications for its cancer chemopreventive potential. Semin Cancer Biol 2016;40–41:141–59. [DOI] [PubMed] [Google Scholar]
- 157. Gueraud F, Tache S, Steghens JP, Milkovic L, Borovic-Sunjic S, Zarkovic N, Gaultier E, Naud N, Helies-Toussaint C, Pierre F et al. . Dietary polyunsaturated fatty acids and heme iron induce oxidative stress biomarkers and a cancer promoting environment in the colon of rats. Free Radical Bio Med 2015;83:192–200. [DOI] [PubMed] [Google Scholar]
- 158. Cui Y, Chen X, Liu L, Xie W, Wu Y, Wu Q, Wang D. Gas chromatography-mass spectrometry analysis of the free fatty acids in serum obtained from patients with Alzheimer's disease. Biomed Mater Eng 2015;26(Suppl 1):S2165–77. [DOI] [PubMed] [Google Scholar]
- 159. Kuratko CN, Salem N Jr.. Biomarkers of DHA status. Prostaglandins Leukot Essent Fatty Acids 2009;81(2–3):111–8. [DOI] [PubMed] [Google Scholar]
- 160. Kasai H, Kawai K. 4-Oxo-2-hexenal, a mutagen formed by omega-3 fat peroxidation: occurrence, detection and adduct formation. Mutat Res 2008;659(1-2):56–9. [DOI] [PubMed] [Google Scholar]
- 161. Prisacaru AE. Effect of antioxidants on polyunsaturated fatty acids—review. Acta Sci Pol Technol Aliment 2016;15(2):121–9. [DOI] [PubMed] [Google Scholar]


