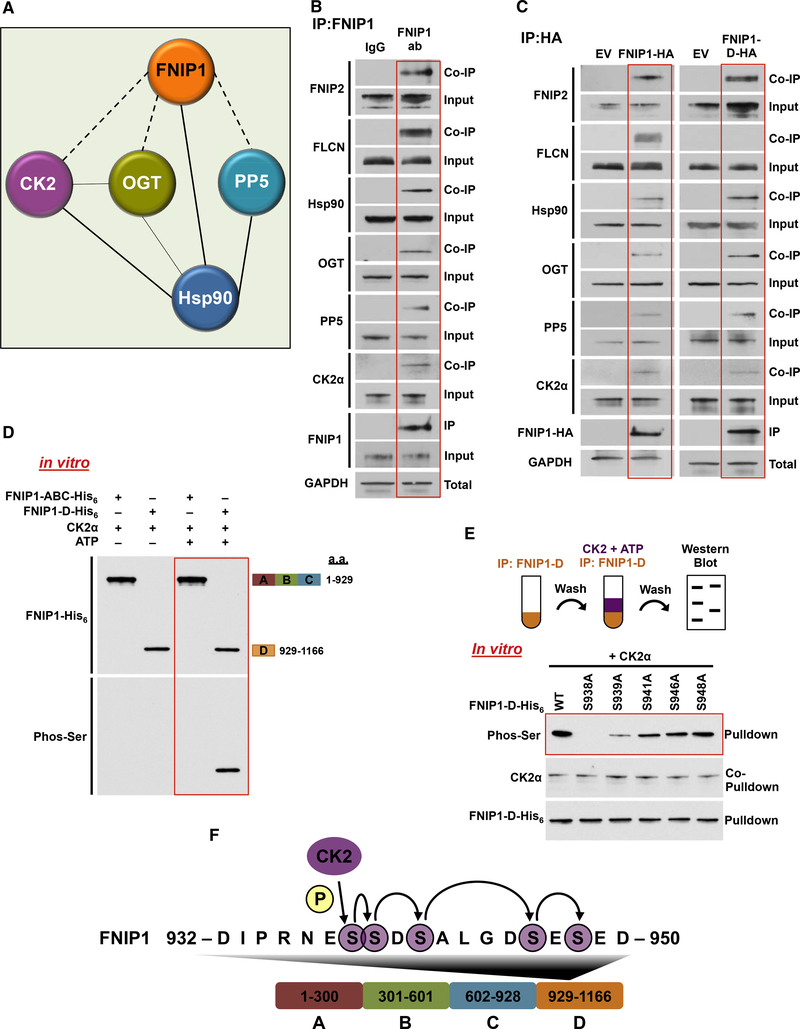
Figure 1. Sequential Phosphorylation of the FNIP1 Co-chaperone by CK2 Kinase.
(A) Endogenous FNIP1 protein was isolated from HEK293 cells, and the profile of interacting proteins was determined by MALDI-TOF.
(B) FNIP1 was immunoprecipitated (IP) from HEK293 cell lysates using anti-FNIP1 or immunoglobulin G (IgG) (control) and immunoblotted with indicated antibodies to confirm protein interactions.
(C) FNIP1-HA, FNIP1-D-HA, and empty vector (EV) were transiently expressed and isolated by IP from HEK293 cells. Indicated coIP proteins were immunoblotted with indicated antibodies to confirm protein interactions.
(D) Indicated FNIP1-His6 fragments were used as substrates of CK2α in an in vitro kinase assay. Phosphorylation of serine residues was assessed by immunoblotting using a pan-anti-phosphoserine antibody.
(E) FNIP1-D-His6 and the indicated non-phosphomutants were bacterially expressed and purified. These proteins were used in an in vitro kinase assay with CK2α kinase. Serine phosphorylation was detected by immunoblotting using a pan-anti-phosphoserine antibody.
(F) Schematic representation of the relay phosphorylation of serine residues in the FNIP1-D fragment.