Fig. 2. Estrogen receptor 1 signaling in wild-type mice and in various transgenic mouse models used to study actions of ESR1.
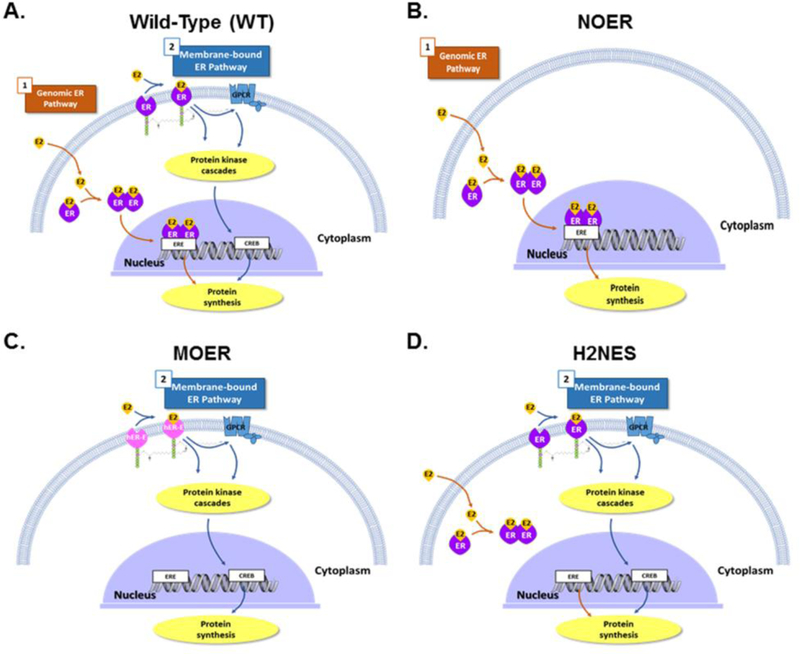
A) In wild-type (WT) mice, genomic estrogen receptor 1 (ESR1,ER) signaling occurs when estrogens bind to cytoplasmic/nuclear receptors, dimerize and then bind to estrogen response element (ERE) within target gene promoter regions and generally induce gene transcription that ultimately leads to translation and increase expression of specific proteins. In the second (non-genomic) pathway for estrogen receptor 1 responses, estrogens bind to membrane estrogen receptor (mESR1,ER), where they can stimulate several intracellular protein kinase cascades and/or G-protein coupled receptors (GPCR), which can result in enhanced cAMP response element-binding protein (CREB)-induced protein transcription, as well as activation of the phosphoinositol 3-kinase/ protein kinase B (PI3K/AKT) and mitogen-activated protein kinase (MAPK) pathways. In addition, 17β-estradiol (E2) or other estrogens could also bind and signal through membrane G protein-coupled estrogen receptor (GPER), which also results in increases in cAMP as well as increased release of epidermal growth factor (EGF) family ligands. In panel A, as well as all the other panels, nuclear and membrane ESR2, as well as GPER, would also be present, but these are not shown in the interest of simplicity. B) Nuclear-only ESR1 (NOER) mice lack membrane ESR1 due to a point mutation in the palmitolyation site (C451A, equivalent site in hESR1 would be Cys447) on this molecule, but the nESR1 pathway remains functional. C) Membrane-only ESR1 (MOER) mice lack both the nESR1 and mESR1 pathways, but a transgene consisting of the E-domain of human ESR1 (hER-E) and another protein fragment that directs all of the protein encoded by this transgene to the membrane has been knocked into these mice, and this mouse has the E-domain of human ESR1 expressed in the plasma membrane. D) Transgenic mice that retain non-genomic ESR1 signaling but lack genomic ESR1 signaling due to mutation in the hinge region of ESR1 (H2NES) possess mutations in the estrogen receptor 1 D-domain, which prevents retention of estrogen-bound ESR1 in the nucleus and thus blocks the classic nuclear pathway involving ligand-bound ESR1 associating with ERE in target genes. Signaling through mESR1 should be unaffected in this transgenic mouse model.
