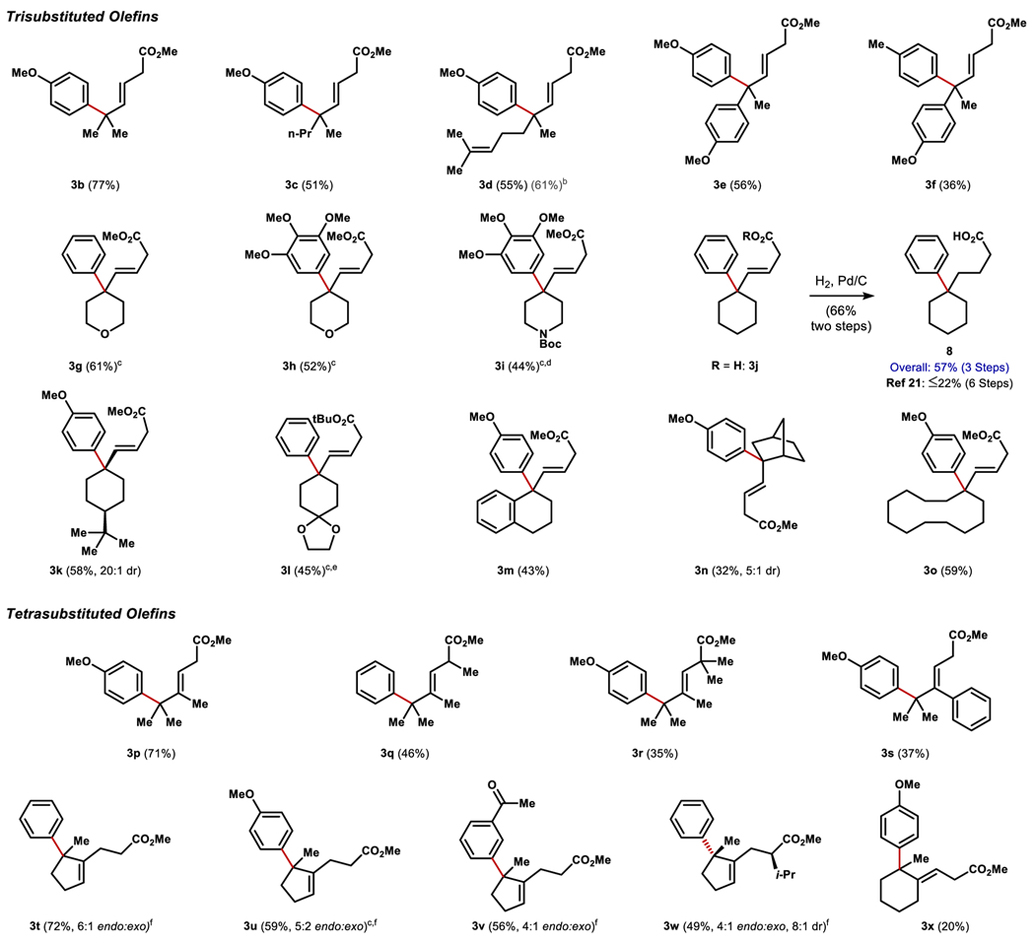
Table 2.
Olefin Scope

0.1 mmol scale, isolated yield over 2 steps, reaction time 16 h. Esterification conditions unless otherwise noted: (COCl)2 (2 eq), DCM (0.1M); MeOH (excess).
1H NMR Yield of crude material.
1 eqivalent of ArBr used.
Esterification: DIC, DMAP, MeOH, DCM.
Esterification: N,N’-diiPr-O-tBu isourea, DCM. [f] Ratio of endocyclic to exocyclic olefin products.