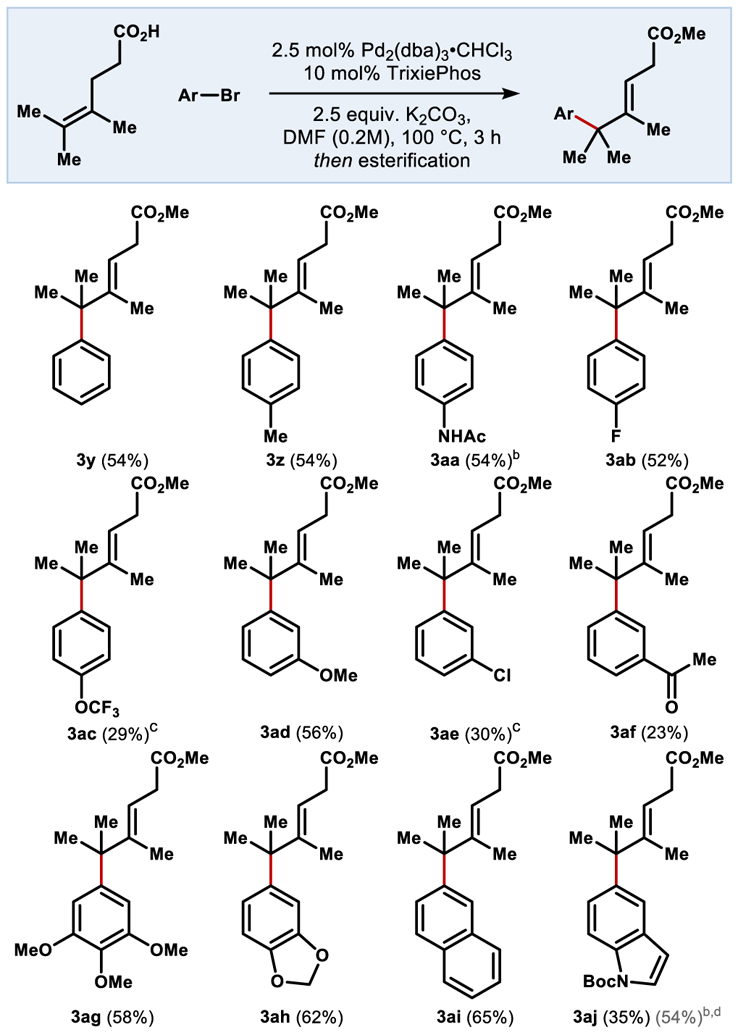
Table 3.
Aryl halide scope with a tetrasubstituted alkene.
0.1 mmol scale, isolated yield over 2 steps, reaction time 16 h. Esterification conditions unless otherwise noted: (COCl)2 (2 eq), DCM (0.1M); MeOH (excess)
Esterification conditions: DIC, DMAP, MeOH, DCM
12:1 ratio of isomers favoring the internal [d] Combined yield including deprotected product.