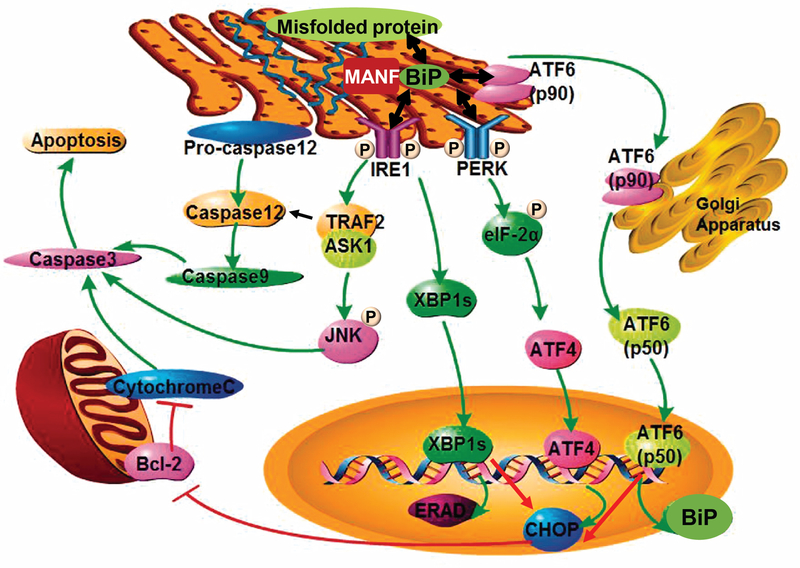
Figure 1: ER stress and the UPR pathway.
Aberrant protein conformations are a major cause of ER disease. A mismatch between the load of unfolded and misfolded proteins and the folding capacity of the ER leads to ER stress. The ER responds to stress by activating intracellular signaling pathways, collectively known as the UPR, which provides a response that aligns cellular physiology to the demands imposed by ER stress. Three different classes of ER stress transducers including IRE1, PERK, and ATF6 have been identified. These proximal sensors are integral membrane proteins, having a domain in the ER lumen, which senses ER stress, and a cytosolic domain, which activates downstream signaling effectors. Three distinct signaling branches IRE1-Xbp1s or IRE1-TRAF2-JNK/caspase 12, Perk-eIF2α-ATF4 and p90ATF6-p50ATF6, are subsequently activated. Cells rendered dysfunctional due to severe or chronic ER stress are eliminated from the organism by ER stress-specific apoptosis, mediated by ER-resident caspase 12, JNK or CHOP. MANF binds to BiP inside the ER, and the interaction is calcium-dependent. The image is modified from Xu et al. [55] with permission.