Figure 1.
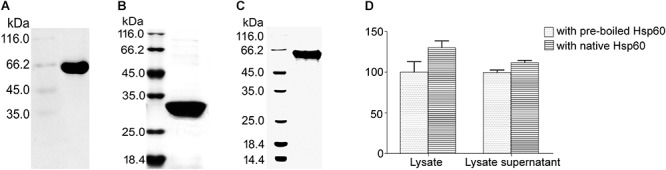
Impact of Hsp60 on the urease activity. (A) Purification of recombinant Hsp60 fused with a 6 × His tag on its C-terminus. (B) UreA fused with a His tag on its C-terminus. (C) UreB fused with a His tag on its C-terminus. The purification of proteins fused with His tags was performed routinely: The supernatant of disrupted bacteria was passed through the Ni-NTA resin at a flow rate of 0.5 ml/min. After being washed with Tris-HCl (50 mM, pH 8.0), the beads were eluted with 250 mM imidazole. For Hsp60, it was further purified with Sephadex G-75 resin at a flow rate of 1 ml/min in HEPES buffer (for urease activity assay) or Tris-HCl (for pull-down assay), or PBS buffer (for SPR analysis). (D) Interaction confirmation by examining the impact of Hsp60 on urease activity of Helicobacter pylori lysate. H. pylori cells were washed and subsequently resuspended in 50 mM HEPES buffer (pH 7.5) to 109 CFU/ml for sonication. The lysate (containing unbroken cells and membrane fractions) or the lysate supernatant (90 μl) was mixed with 100 μl of Hsp60 (0.2 mg/ml), followed by incubation at 37°C for 30 min. Then 100 μl of urea solution (62.5 mM in HEPES buffer) was added and incubated for another 30 min at 37°C. The reaction was stopped by adding 375 μl of regent A (containing 10 g/l phenol and 50 mg/l sodium pentacyanonitrosylferrate(III) dihydrate) and 375 μl of regent B [containing 5 mg/ml sodium hydroxide, 0.044% (v/v) sodium hypochlorite] successively. After a further 30-min reaction at 37°C, the absorbance at 620 nm was measured. The activity of lysate or lysate supernatant with pre-boiled Hsp60 was taken as control (100%). All experiments were repeated at least three times.
