Figure 2.
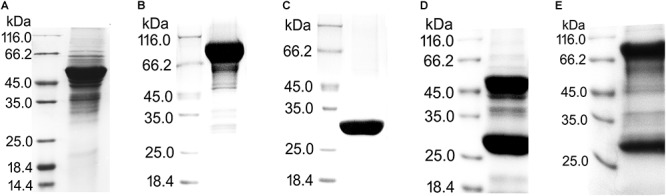
Purification of recombinant proteins with GST tags for pull-down assays. (A) UreA fused with GST tag on its N-terminus (GST-UreA). (B) UreB fused with GST tag on its N-terminus (GST-UreB). (C) GST tag. (D) UreA fused with GST tag on its C-terminus (UreA-GST). (E) UreB fused with GST tag on its C-terminus (UreB-GST). The recombinant proteins with GST tags were all purified as follows: The bacterial cells were disrupted in Tris-HCl (50 mM, pH 8.0) and centrifuged at 11,000 rpm for 30 min. The supernatant was then incubated with glutathione Sepharose 4B beads overnight with gentle inversion. Next, the beads were washed three times with Tris-HCl (50 mM, pH 8.0) and eluted with glutathione (reduced form) to a final concentration of 10 mM. During the purification of UreA-GST and UreB-GST, a large amount of cleaved GST tag (about 26 kDa) was also eluted from the beads.
