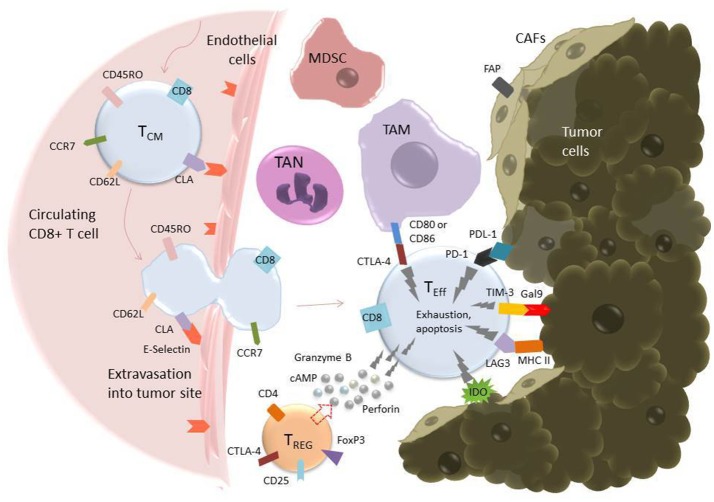
Figure 2.
T cell extravasation into the TME and subsequent exhaustion mediated by inhibitory ligands on tumor and tumor-associated cells. Endothelial cells experiencing inflammation express adhesion molecules including selectins, vascular cell adhesion molecule-1 (VCAM-1), and intercellular adhesion molecule (ICAM-1). P- and E-Selectins (the latter shown in the figure) bind cutaneous lymphocyte antigen (CLA), a specially glycosylated form of P-selectin glycoprotein ligand 1 (PSGL-1) that is expressed on activated T cells (114). VCAM-1 binds very late antigen-1 (VLA-4) and ICAM-1 binds lymphocyte function-associated antigen-1 (LFA-1) (115). Upon binding endothelial cell ligands, T cells undergo tethering and rolling before adhering to the endothelium and transmigrating through it as shown. Once in the tumor microenvironment, T cells are in an environment full of tumor-associated, immunosuppressive cells including tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), myeloid-derived suppressor cells (MDSCs), T-regulatory cells (Tregs), and cancer-associated fibroblasts (CAFs) (95). These cells express inhibitory molecules, including CD80/CD86, which bind the inhibitory receptor CTLA-4 (pictured), and secrete soluble factors that suppress or cause apoptosis in T cells. CAFs also serve as a physical barrier between T cell and tumor cell. Additionally, tumor cells themselves express ligands such as Gal9 and PDL-1, which bind to the T cell inhibitory receptors TIM-3 and PD-1, respectively. All these factors serve to promote an “exhausted” phenotype in the T cell, characterized by upregulation of inhibitory receptors such as PD-1, TIM-3, TIGIT, and LAG-3, loss of CCR7, CD62L, and CD45R0, loss of cytotoxicity, and apoptosis (111).